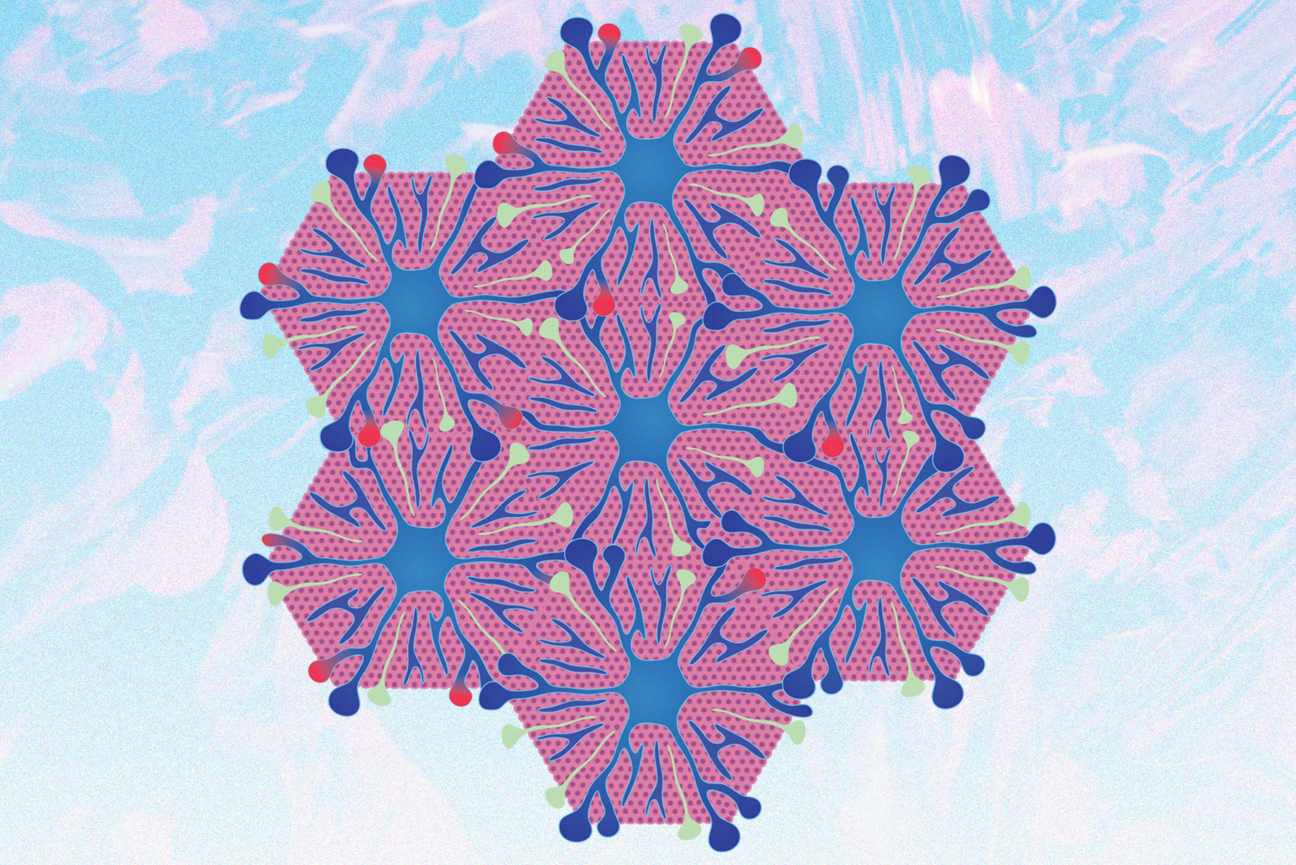
Liver cells assume traits and functions according to their location within hexagonal arrangements around veins within the liver.
Madeleine Turner/Whitehead Institute
Biology in its natural habitat
This article is part of our series, “Biology in its natural habitat: studying cellular processes in context”. To view the entire collection, click here.
Researchers at Whitehead Institute are developing models and approaches that capture more of the rich context in which biological processes are typically immersed, in order to learn more about them and their roles in health and disease. Read on to learn more about how their work is revealing new insights that only become apparent when studying a process within the context of larger systems in which it operates.
A cell's environment is important for its identity
Whitehead Institute Founding Member Rudolf Jaenisch works with induced pluripotent stem cells (IPSCs). These are adult cells that have been manipulated to turn back into stem cells, immature cells that are capable of maturing into any almost other cell type when exposed to the right combination of signals. IPSC-derived cells can provide an otherwise difficult to obtain source of cells for gaining insights into diseases that affect particular cell types.
For researchers to make IPSC-derived cell types, they must identify the combinations of signals that cause stem cells to mature into the desired cell type in the body. The more closely the researchers can replicate the signals present in the body, the more closely IPSC-derived cells will resemble the body’s cells. For example, Jaenisch lab researchers figured out how to make IPSC-derived liver cells more closely resemble adult liver cells by identifying the important role that a thyroid hormone plays in liver cell maturation. Other Jaenisch lab researchers found that fat cells grown in media containing concentrations of insulin and glucose similar to those in the human body will accurately model healthy or diabetic insulin response, whereas cells grown in typical lab media will not.
Thyroid hormone receptor beta (THRB) helps an immature liver cell get to its destination, a mature state.
Andrew Tubelli
Jaenisch lab researchers often grow cells in three dimensional assemblies that resemble real tissues, as this can lead to better results than growing cells in typical 2D cultures. 3D cultures, often called organoids because they resemble miniature organs, can be structured to replicate some aspects of the cells’ natural environment in ways that are not possible in two dimensions, such as blood flow and 3D spatial organization within a tissue. Making sure that stem cell models closely resemble cells within the body is important because the less accurate the model is, the less likely that it will provide results that are relevant to human health and disease.
Studying cellular interactions can show us how diseases can spread
Jaenisch lab researchers have also found that growing different cell types together can be beneficial. When they were trying to solve why Covid-19, a respiratory illness, often caused vascular symptoms (symptoms related to blow flow), they used IPSCs to grow vascular cells along with two neighboring cell types.
They found that although the SARS-CoV-2 virus that causes Covid-19 does not infect vascular cells, it affects them indirectly by infecting neighboring cells. Communication between the infected neighbors and uninfected vascular cells causes changes in vascular cells that appear to be responsible for Covid-19’s vascular symptoms. If the researchers had studied the vascular cells in isolation, they would only have observed the virus failing to infect the cells, and not have gained these insights into Covid-19’s vascular effects.
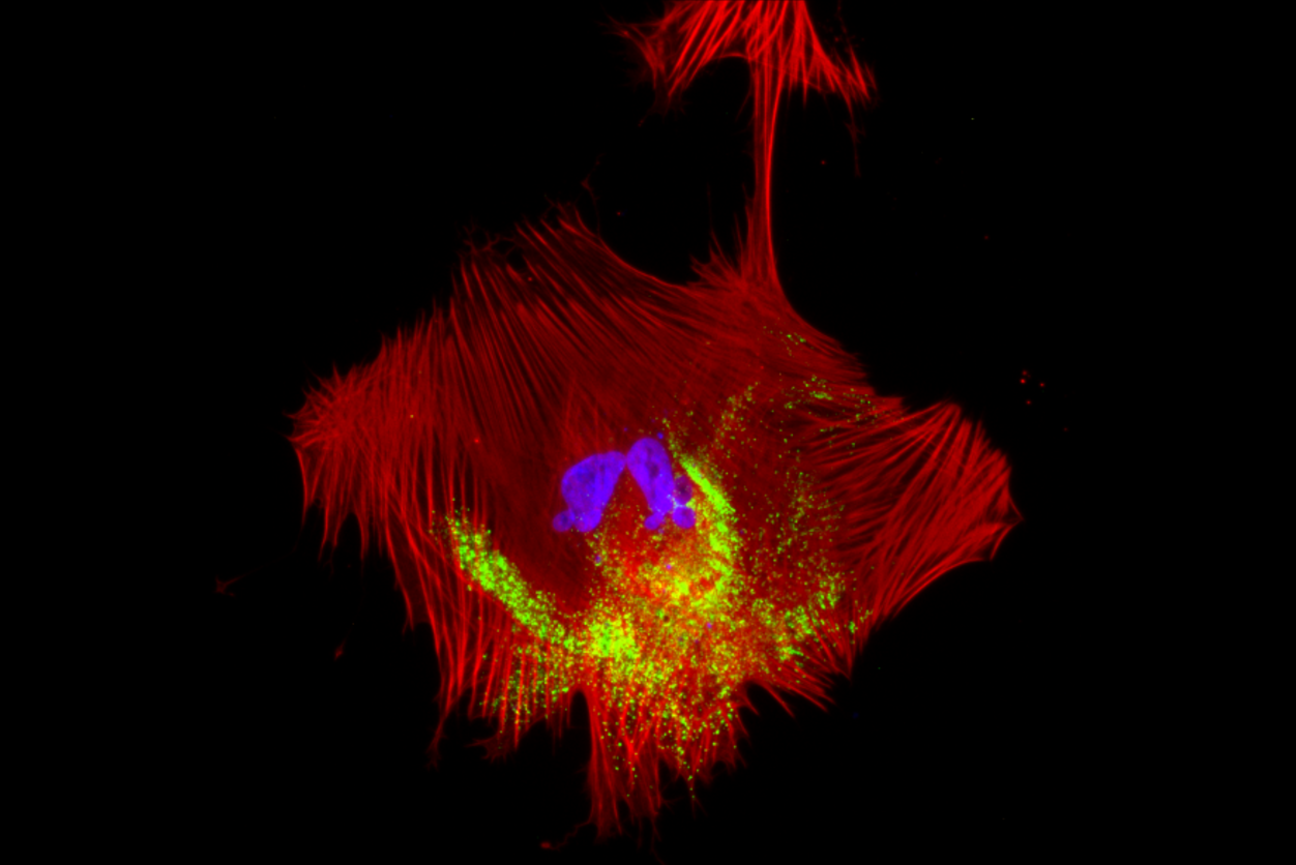
A human pluripotent stem cell-derived smooth muscle cell infected with the virus SARS-Cov-2. Sites of viral genome replication are in green.
Alexsia Richards/Whitehead Institute
Genes in context
In the same way that no cell operates in isolation, no gene works alone. The activity of a gene—how much of its protein product gets made—is regulated by many different things, including DNA sequences called enhancers that can increase the activity of a target gene. Whitehead Institute Member Olivia Corradin analyzes genes in their regulatory context to figure out how small changes in them or in their regulatory network contribute to disease. Looking at the whole regulatory network instead of the gene in isolation can reveal important information.
For example, this is how Corradin and colleagues discovered that oligodendrocytes, a type of brain cell that creates the insulation that wraps around neurons, appear to play a role in the autoimmune disease multiple sclerosis (MS). Previously, genes that contribute to multiple sclerosis had been identified through genome wide association studies (GWAS), in which researchers compare genetic data from patients with and without multiple sclerosis to identify genetic differences that are present primarily in people with MS. On average, the genes implicated are most active in immune cells, suggesting immune cells are the main actors in the disease. However, that doesn’t tell the full story.

Steven Lee/Whitehead Institute
Corradin set out to identify the specific cell type through which each MS-linked gene contributed to the disease. She did this by mapping the networks of enhancers that regulate MS-linked genes, and looked at the whole networks’ association to disease risk. Although the same gene may be active in many different types of cells, the regulatory network is likely to be specific to one cell type. Therefore, the approach reveals disease-relevant cell types. The approach identified immune cells, but it also pointed to other cell types, including oligodendrocytes. Further experiments revealed a role for oligodendrocytes in MS, demonstrating the value of analyzing genes in the context of their regulatory networks.
Corradin and colleagues applied a similar approach to identify genes that may contribute to opioid use disorder (OUD), revealing patterns that were not visible when looking at the genes on their own. Corradin is now building on her OUD work, looking at gene expression changes in the context of different environmental exposures such as opioid exposure and high stress levels.
Capturing lots of different data at once to get a fuller picture
Animal models provide opportunities to observe cells in context, but there are limitations to what a researcher can actually see or measure in a living organism. Researchers in Whitehead Institute Member Jonathan Weissman’s lab, along with Harvard University Professor Xiaowei Zhuang and Stanford University School of Medicine Assistant Professor Will Allen, have developed a screening approach called Perturb-Multi that collects and combines multiple types of data from tissue samples to capture as much information as they can about cells from inside a mouse. The goal of the approach is to analyze cells in as close to a living state as possible, and synthesize data about their gene expression, contents, and relationships with other cells. Getting this unprecedented, detailed look at cells within tissues will provide new insights into many biological processes. The researchers have applied their tool to the liver, and gained insights into how liver cell identity is regulated, how fat accumulates in liver cells, and more.
Madeleine Turner/Whitehead Institute
One key step of Perturb-Multi is that the researchers fix and preserve cells and sections of tissue in the state they were in when they were collected. This is important because, in the same way that stem cells struggle to assume the identity of a specific cell type when grown outside of a body, adult cells taken out of a body quickly lose many of their characteristics and functions. For example, liver cells arrange themselves into hexagonal structures around veins, and assume identities with different gene expression and function based on how close they are to the center. When liver cells are grown or put in a dish, they lose their zone-based identities. Perturb-Multi allows researchers to study liver cells with intact zonation.
Other key steps of Perturb-Multi are that the researchers collect the gene expression information of individual cells and take images of the tissue in which the cells reside. The images show where cells are in relation to each other, as well as the structures and molecules of interest inside of each cell. Synthesizing the gene expression and imaging data allows researchers to draw new connections. For example, a common health problem in the liver is that cells start accumulating fat in the form of lipid droplets. This can contribute to liver disease. The researchers manipulated cells in the mouse liver by turning off single genes in individual cells and then observing the effects. From the images they collected, they found four genes that, when turned off, led to the visible accumulation of lipid droplets in those cells. However, when they looked at the gene expression data from the cells, they found that the droplet accumulation was being driven by three different mechanisms. Biological insights like this could be very useful for medicine down the road: for example, liver patients could be profiled based on what genetic changes are driving their liver disease, and given treatments that are targeted to the specific pathway by which fat is accumulating in their livers.
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


