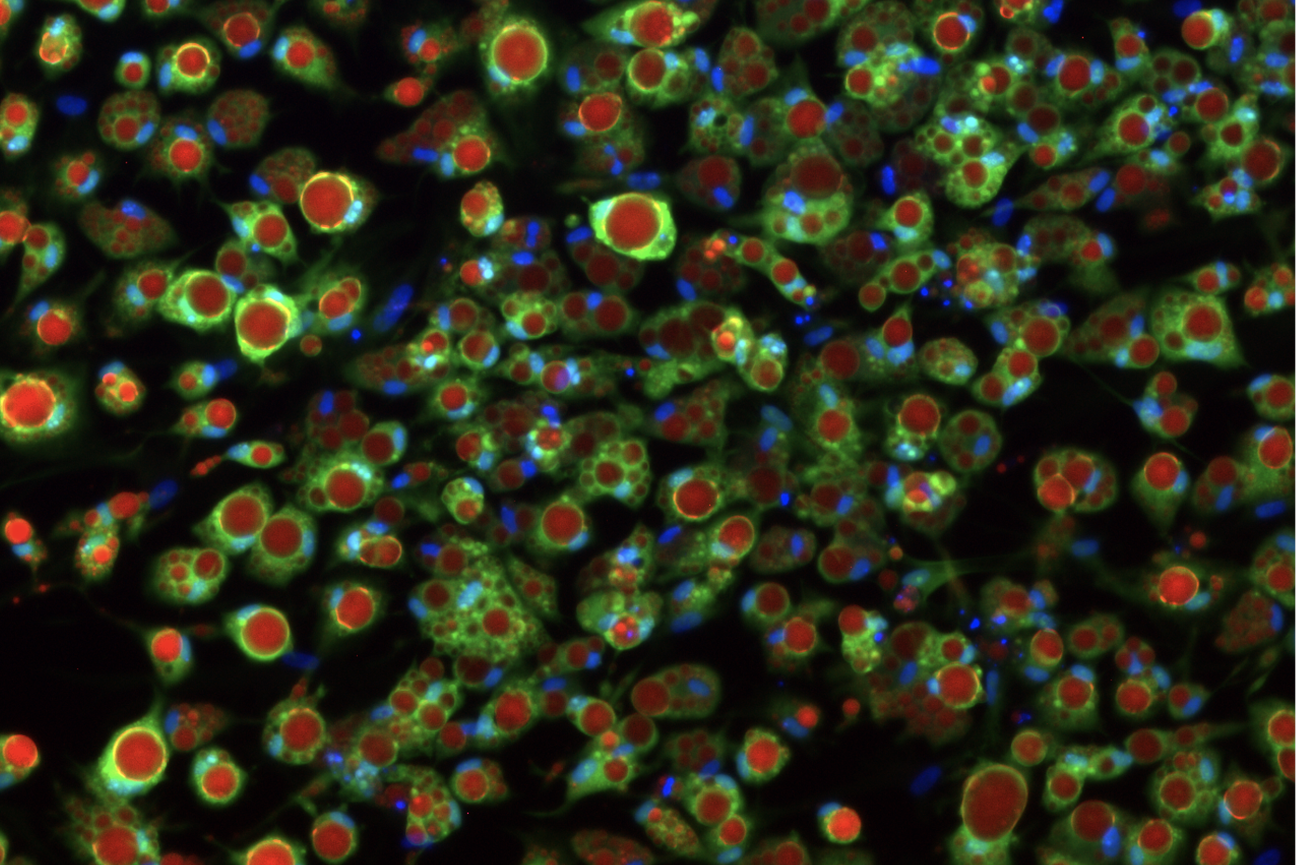
Lab-grown adipocyte with nuclei in blue, green being Cellmask staining the whole cell membrane, and in red the distinguishing features of adipocytes - lipid droplets - stained with Lipidtox.
Courtesy of Max Friesen
Lab-grown fat cells help scientists understand type 2 diabetes
In research published June 17 in the journal Science Advances, researchers in the lab of Whitehead Institute Founding Member Rudolf Jaenisch present a way to create fat cells that can be modified to display different levels of insulin sensitivity.
The cells accurately model healthy insulin metabolism, as well as insulin resistance, one of the key hallmarks of type 2 diabetes. “This system, I think, will be really useful for studying the mechanisms of this disease,” said Jaenisch, who is also a professor of biology at the Massachusetts Institute of Technology (MIT).
“It's really exciting,” said Max Friesen, a postdoctoral researcher in Jaenisch’s lab and a first author of the study. “This is the first time that you can actually use a human stem cell-derived [fat cell] to show a real insulin response.”
Body fat — also known as adipose tissue — is essential for regulating your body’s metabolism and plays an important role in the storage and release of energy. When fat cells called adipocytes encounter the hormone insulin, they suck up sugar from the blood and store it for future use.
But over many years, factors such as genetics, stress, certain diets, or polluted air or water can cause this process to go awry, leading to type 2 diabetes. In this disease, adipocytes, as well as cells in the muscles and liver, become resistant to insulin and therefore unable to regulate the levels of sugar in the blood.
Tools to model diabetes in the lab generally rely on mice or on cells in a petri dish or test tube. Both these systems have their own problems. Mice, although they are comparable with humans in some respects, have a completely different metabolism and do not experience human diabetes comorbidities like heart attacks. And cell culture has, in the past, failed to replicate key markers of diabetes in a way that is comparable to human tissues.
That’s why Friesen and Andrew Khalil, another postdoc in Jaenisch’s lab, set out to create a new model. The researchers started with human pluripotent stem cells. These cells are the shapeshifters of the body — given the right conditions, they can assume the specific characteristics of almost any human cell type. The Jaenisch Lab has used them in the past to replicate liver cells, brain cells, and even cancerous tumors.
They decided to try to optimize an existing method for differentiating pluripotent cells into fat cells. The protocol created cells that looked like adipocytes, but these cells did not recreate the conditions of healthy insulin signaling or insulin resistance seen in the human body in type 2 diabetes. When healthy adipocytes encounter insulin in the human body, they respond by taking up glucose out of the bloodstream. These lab-made fat cells weren’t doing that, unless the researchers cranked up insulin levels to a thousand times higher than levels ever seen in humans. “Taking up glucose [in response to normal levels on insulin] is really the main function of an adipocyte, so if the model fails to do that, anything downstream in terms of disease research is not going to work either,” Friesen said.
Friesen and Khalil wondered if the lab-grown adipocytes’ low sensitivity to insulin could be a product of the conditions in which they grew. “We thought that maybe this happens because we're feeding them an artificial culture medium, with all kinds of extra supplements that might be inhibiting their metabolic response,” Friesen said.
Friesen and Khalil decided to use a method called the Design of Experiments approach, which allows researchers to tease out the contributions of different factors to a specific outcome. Informed by this approach, they created nearly 30 different media compositions, each with slightly different levels of key ingredients such as glucose, insulin, the growth factor IGF-1, and albumin, a protein found in blood serum.
The medium that worked best had concentrations of insulin and glucose that were similar to the levels in the human body. When grown in this new medium, the cells responded to much lower concentrations of insulin, just like cells in the body. “So this is our healthy adipocyte,” Friesen said. “Next we wanted to see if we could make a disease model out of this — to make it an insulin-resistant adipocyte like you would see in the progression to type 2 diabetes.”
To desensitize the cells, they flooded the media with insulin for a short period of time. This caused the cells to become less sensitive to the hormone, and respond similarly to diabetic or pre-diabetic fat cells in a living person.
The researchers could then study how the cells responded to the change — such as what genes the insulin resistant cells expressed that healthy cells did not — in order to tease out the underlying genetics of insulin resistance. “We saw small changes in a lot of genes that are metabolism regulated, so that seems to be pointing to a deficiency of the metabolism or mitochondria of the insulin-resistant cells,” Friesen said. “That's one thing we want to pursue in the future — figure out what is wrong with their metabolism, and then hopefully how to fix it.”
Now that they have created this new model for studying insulin resistance in fat cells, the researchers hope to develop similar procedures for other cells affected in diabetes. “It seems that with some modifications, we can apply this method to other tissues as well,” Friesen said. “In the future, this will hopefully lead to a unified system for all stem cell-derived tissues, including liver, skeletal muscle, and other cell types, to get a really robust insulin response.”
Max Friesen, Andrew S. Khalil, M. Inmaculada Barrasa, Jacob F. Jeppesen, David J. Mooney, Rudolf Jaenisch. "Development of a physiological insulin resistance model in human stem cell-derived adipocytes." Science Advances, June 17, 2022. DOI: 10.1126/sciadv.abn729
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu

