Transcriptional droplets provide evidence for new model of gene control
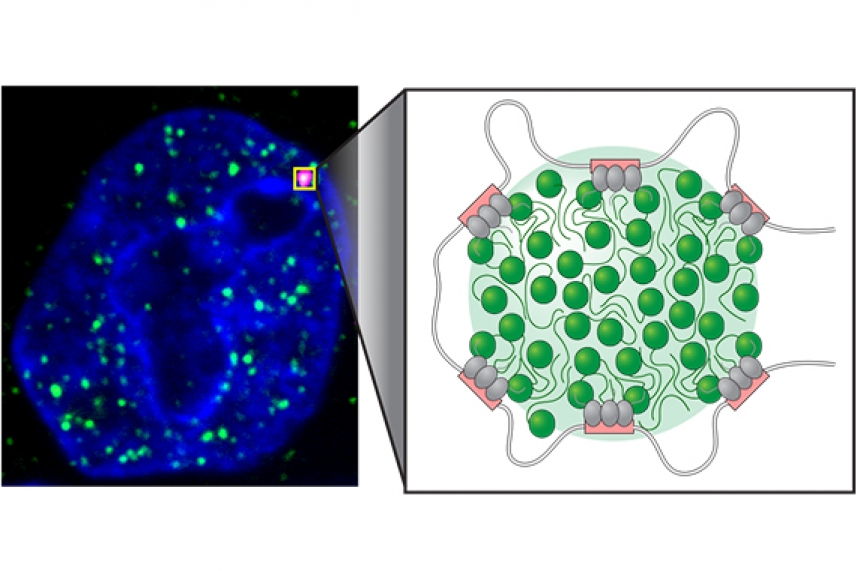
Image of transcriptional condensates (left). Model of transcriptional condensate with super-enhancer elements (pink), transcription factors (gray), and transcriptional components (right; green).
Alessandra Dall'Agnese and Benjamin Sabari/Whitehead Institute
Cambridge, MA. – Whitehead Institute scientists have found evidence that the apparatus that transcribes DNA into RNA is concentrated into specialized droplets, or “condensates”, at genes that control cell identity. These condensates, which are formed in the absence of membranes, appear to concentrate the transcription apparatus and ensure that key cell identity genes are efficiently transcribed. The identification and characterization of these transcriptional condensates may have implications for cellular control in health and disease and may lead to new therapeutic approaches to diseases such as cancer.
“These findings challenge our conventional views of gene control, how the genome is organized, and the fundamental operating systems of cells” says Whitehead Member Richard Young, who is also a professor of biology at Massachusetts Institute of Technology (MIT).
At the core of this work, which was described online June 21 in the journal Science, is a special type of gene regulatory element called a super-enhancer. Super-enhancers are large regulatory regions in the genome that control genes with prominent roles in the identity of each type of cell, such as brain, skin, or liver, in people. Super-enhancers also play important roles in disease; cancer cells find ways to create super-enhancers at the oncogenes that drive their tumor identity. Despite the central roles of super-enhancers in normal and disease biology, why and how they operate at the most important genes has been something of a mystery.
Young and members of his lab at Whitehead Institute and MIT recently hypothesized that super-enhancers may form transcriptional condensates that could concentrate large amounts of transcription apparatus at key genes, which would then allow unfettered synthesis of RNA from these genes. An important clue that led to this hypothesis is knowledge that certain proteins, including the protein machinery involved in transcription, have a feature that promotes formation of bodies that are known by physicists as “phase-separated condensates”. Proteins, which are the molecular machines of cells, fold into various three-dimensional forms, some of which are structurally quite stable. Other forms, which move about constantly, are unstable and dynamic. It is this feature of unstable and dynamic protein structure that can promote formation of condensates.
In the current work, Alessandra Dall'Agnese and Benjamin Sabari, postdoctoral researchers in the Young lab, led a team to test the hypothesis that super-enhancers form condensates that could concentrate large amounts of transcriptional apparatus at key genes. The duo used super-resolution microscopy to reveal dense bodies containing vital transcriptional components at super-enhancers in the genomes of embryonic stem cells, and found that transcriptional components could move rapidly through the bodies, a feature consistent with condensates. They showed that these same transcriptional components could form condensates in test tubes in their purified forms and identified the amino acid constituents that were responsible for this condensate behavior.
Sabari and Dall’Agnese then investigated whether these test tube condensates could also incorporate other proteins necessary for transcription. When introduced into a cell extract containing the large array of proteins necessary for transcription in living cells, many of those proteins were incorporated into the droplets and concentrated within them.
“Our work suggests that these condensates are a way to compartmentalize and concentrate factors at particular locations within the cell,” says Sabari. “Although we’ve known about other kinds of condensates within the cell, this is the first link between condensates and the control of cell identity genes.”
Young likens each condensate to an address at which all of the proteins involved in a process can congregate. Instead of molecules wandering around the nucleus until they bump into their transcriptional colleagues, the interacting proteins create a mesh within the condensate that collects the right components at the right place for the transcription of key cell identity genes. Bringing all of the diverse molecules needed for this multistep process together increases the efficiency of transcription. Condensates containing high densities of factors at super-enhancers could also ensure that a cell never runs out transcriptional supplies at these important genes.
The new model could have implications for disease and new therapeutic concepts. “Disease-associated genetic variation has been noted in super-enhancers, and this new model makes us wonder if such variation impacts condensate regulation”, says Dall’Agnese. “We also wonder if condensates might influence how drugs reach their targets.”
Sabari and Dall’Agnese’s work upends Young’s perspective on the control of transcription and its relevance to disease.
“It’s interesting to consider whether the biophysical properties of condensates may be responsible for other observations in regulatory biology,” says Young. “This concept provides an opportunity to see regulation of normal and disease cells in a new light.”
This work was supported by National Institutes of Health (NIH grants GM123511 and P01-CA042063), the National Science Foundation (NSF grant PHY-1743900), the National Cancer Institute (P30-CA14051), Damon Runyon Cancer Research Foundation (2309-17), Swedish Research Council (VR 2017-00372), Hope Funds for Cancer Research, Cancer Research Institute, American Cancer Society New England Division (PF-16-146-01-DMC), and the Netherlands Organisation for Scientific Research (NWO).
***
Richard Young’s primary affiliation is with Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a professor of biology at Massachusetts Institute of Technology.
***
Citation
Sabari, B. R.*, Dall’Agnese, A.*, Boija, A., Klein, I. A., Coffey, E. L., Shrinivas, K., ... & Young, R. A. (2018). Coactivator condensation at super-enhancers links phase separation and gene control. Science, 361(6400).
*Co-first authors
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


