Scientists identify mTOR pathway mutations in follicular lymphoma
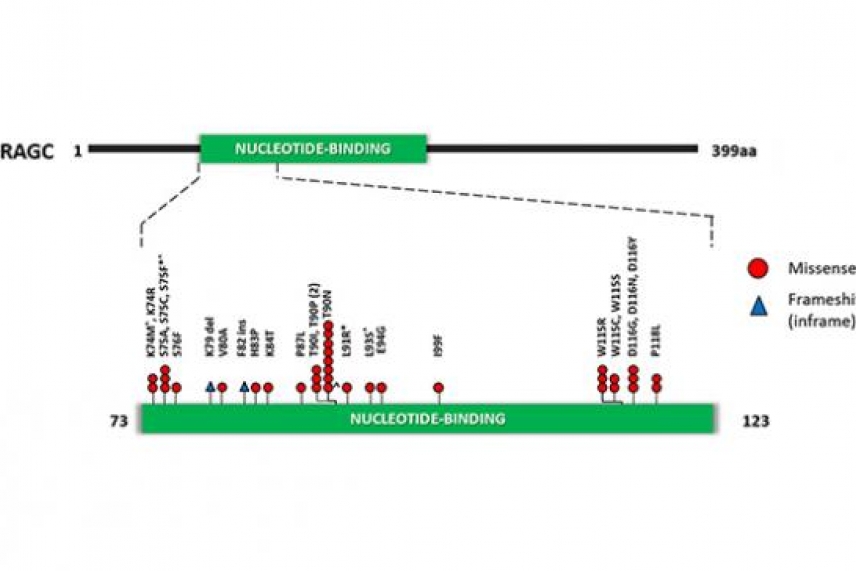
The gene encoding the protein RagC is often mutated in the tumors of follicular lymphoma patients. This diagram shows the frequency of each mutation in the gene. RagC mutations could serve as a biomarker to predict therapeutic response.
Courtesy of Nature Press
CAMBRIDGE, Mass. (December 21, 2015) – A team of researchers from Whitehead Institute and Queen Mary University of London (QMUL) have identified in follicular lymphoma patient tumors mutations in the RRAGC gene that could serve as a biomarker to predict therapeutic response.
The research is reported online this week in the journal Nature Genetics.
One of the most common non-Hodgkin lymphomas, follicular lymphoma is characterized by uncontrolled growth and multiplication of the B cells of the immune system. Most patients experience slow disease progression marked by multiple relapses. However, some patients develop a more aggressive form, diffuse large B cell lymphoma (DLBCL)
To understand the genetic causes of follicular lymphoma, Whitehead and QMUL scientists analyzed the mutations found in patient tumors with multiple relapses of follicular lymphoma, without transformation to DLBCL. According to their work, one commonly mutated gene encodes the protein RagC, which is essential for activating the amino-acid sensing mTORC1 (for “mechanistic target of rapamycin complex 1”) pathway. Although mutations in genes in the mTORC1 pathway have been associated with various cancers, this is the first time a genetic mutation in any of the four Rag proteins has been identified in malignancy.
“One of the mutations that we have identified allows follicular lymphoma tumors to turn on growth signals regardless of whether nutrients are available, thereby evading normal restrictions on its growth,” says Jessica Okosun, a scientist at QMUL’s Barts Cancer Institute and co-first author on the Nature Genetics paper. “Remarkably, the mutations we have discovered have not been seen in other cancer types. However, drugs that directly target this nutrient-sensing mechanism are currently used to treat other types of cancer, and may benefit patients with follicular lymphoma.”
In cell lines in culture, the scientists show that expression of the mutated RagC proteins activate mTORC1 signaling in the absence of amino acids and increase binding to an important part of the mTORC1 complex, consistent with the established role of RagC in the mTORC1 pathway. Because this latest research was performed exclusively in cell lines, the scientists have not yet deciphered the mutations’ mechanistic effect in patients. However, Rachel Wolfson, a graduate student in Sabatini’s lab and one of the paper’s co-first authors, says there are clues to the mutations’ significance.
“mTORC1 is linked to cell growth, so it is not surprising that activation of the pathway could lead to some growth advantage for cancer cells,” says Wolfson. “But it leads to an interesting question: When is it a proliferative advantage versus a disadvantage to no longer be able to accurately sense amino acid levels? That is something we would need to investigate further, likely in vivo.”
The researchers would also like to know how the drug rapamycin affects follicular lymphomas with RagC mutations. Rapamycin, an FDA-approved immunosuppressant used to prevent organ transplant rejection, binds to mTORC1 and inhibits its activity. If rapamycin interferes with mTORC1 dysregulation caused by RagC mutations, perhaps the drug could be used in follicular lymphoma treatment.
“If so, maybe these RagC mutations could be used as biomarkers to predict sensitivity to rapamycin treatment in follicular lymphoma patients,” says Wolfson. “That would be very exciting, and it’s something that should be investigated further.”
This work was supported by Barts, Cambridge, Leeds and Southampton’s Experimental Cancer Medicine and Cancer Research UK Centers and the Kay Kendall Leukaemia Fund and Cancer Research UK (grants KKL 757 and 15968).
* * *
David Sabatini's primary affiliation is with Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a Howard Hughes Medical Institute investigator and a professor of biology at Massachusetts Institute of Technology.
* * *
Citation:
Okosun, J., Wolfson, R. L., Wang, J., Araf, S., Wilkins, L., Castellano, B. M., ... & Fitzgibbon, J. (2016). Recurrent mTORC1-activating RRAGC mutations in follicular lymphoma. Nature genetics, 48(2), 183-188.
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


