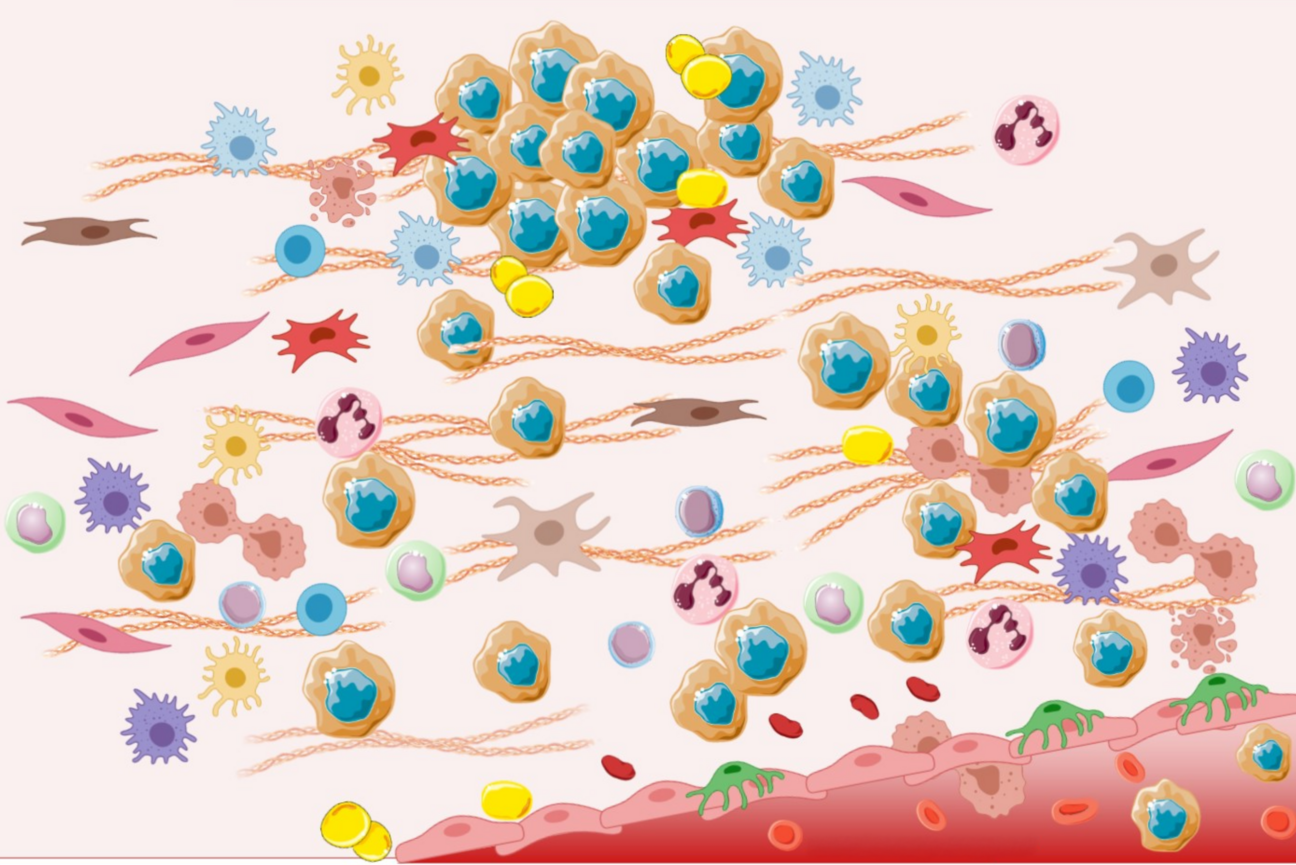
Cancer cells (brown with blue centers) interact with many other types of cells inside of tumors.
Excerpted from Figure 2, "Epithelial to Mesenchymal Transition: A Challenging Playground for Translational Research. Current Models and Focus on TWIST1 Relevance and Gastrointestinal Cancers." Int. J. Mol. Sci. 2021, 22, 11469. https://doi.org/10.3390/ijms222111469. Licensed under CC-BY 4.0.
The company we keep: Revealing cancer’s accomplices
This article is part of our series, “Biology in its natural habitat: studying cellular processes in context”. To view the entire collection, click here.
Tumors are diverse communities of cells that in addition to cancer cells, may contain vascular cells, immune cells, and connective tissue-generating fibroblasts. Often these other cell types help cancer cells to grow, spread, and avoid the immune system and medical therapies. However, under certain conditions, these other cell types can actually come to hinder cancer cells. These relationships can shift depending on signals that the different cell types receive from the cancer cells, the local environment within the tumor––known as the tumor microenvironment––or medical therapies. The ability of cancer cells to co-opt their neighbors and change their microenvironment in their favor is part of the reason that cancer is such a deadly disease and so difficult to treat.
As researchers learn more about how cancer cells interact with other cell types and components of the tumor microenvironment, they not only learn more about how cancer progresses to life-threatening stages, but also discover new opportunities to treat cancer. Just as cancer cells can co-opt other cell types to protect themselves, so can therapies co-opt various cell types to destroy the cancer. Thus, immunotherapy, which manipulates and deploys the body's immune cells, has had substantial success against cancers that previously appeared untreatable.
To create an immunotherapy, researchers identify the signals that cancers usually send to immune cells to prevent the latter from attacking them; by blocking these defensive signals, researchers can unleash the immune cells, enabling them to destroy the cancer cells. Whitehead Institute Valhalla Fellow Kipp Weiskopf works on such immunotherapy strategies: he focuses on CD47, a “don’t eat me” signal molecule that cancer cells use to avoid detection by macrophages, immune cells that otherwise would destroy them. Weiskopf is investigating how to best prime macrophages to destroy cancer cells. In lung cancer, his lab has found that combining therapies that block CD47 signaling with therapies that target cancer cells directly can slow their growth and lead to better success than using either drug in isolation. The Weiskopf lab is now developing new antibody therapies that further leverage these principles for use in patients in the future.
Whitehead Institute Valhalla Fellow Tobiloba Oni studies pancreatic cancer, one of the deadliest and most treatment-resistant cancers. The Oni lab seeks to understand how pancreatic cancer cells manipulate their microenvironment to escape immune attack and resist therapy. Oni frames the tumor as a complex network of interactions and asks a fundamental question: which interactions between cancer cells and their neighbors are positive, neutral, or negative for the cancer cells? A negative interaction might involve an immune cell detecting and destroying a cancer cell. A neutral interaction could be a cell type that coexists with cancer cells but doesn’t alter their fate. A positive interaction, by contrast, actively supports cancer cells—for example, fibroblasts releasing cytokines that help cancer cells resist therapy, or neutrophils suppressing other immune cells, shielding the cancer cells from immune attack. By systematically classifying the nature of these interactions, Oni aims to understand the rules of engagement that allow cancer cells to turn their microenvironment into a source of support.

Kipp Weiskopf (left) and Tobiloba Oni (right)
Gretchen Ertl/Whitehead Institute
Oni is particularly interested in how cancer cells engage in these interactions: what signals do they use to recruit allies from populations of bystanders or even enemy cells? He focuses on a class of molecules called glycans, which are sugar molecules attached to proteins, lipids, and RNA on the cell surface. Researchers have long recognized that cancer cells carry unique glycans, many of which are already used as biomarkers for cancer detection and monitoring. However, these sugars are not just passive markers; they are dynamic communication tools. Oni’s lab is currently investigating how cancer cells use these glycans to generate positive interactions with nearby cells and how these interactions can be targeted to suppress tumor growth.
This work shows how understanding which interactions are positive, neutral, or negative for cancer cells can help researchers find new ways to reprogram these relationships to restrict tumor growth and improve patient outcomes. Weiskopf and Oni expect that combination therapies that target both cancer cells and their neighbors will become more commonly used moving forward. Oni speculates that combination therapies may expand to target many more cell types than cancer and immune cells, as researchers learn to take advantage of the same intercellular relationships that cancer cells currently use to their benefit.
Madeleine Turner/Whitehead Institute
How cancer cells spread to new environments
Cancer cells’ microenvironment and intercellular interactions are important at the original site at which the cancer begins to grow – but they also factor into whether cancer cells can metastasize, or spread and form new tumors at distant sites in the body. Whitehead Institute Founding Member Robert Weinberg studies how breast cancer cells gain the ability to metastasize. This invariably causes these cancers to become deadly.
Weinberg and others have found that a key early step in metastasis is for cancer cells to undergo a change called the epithelial-to-mesenchymal transition (EMT), which enables them to spread. Researchers have found that signals from the tumor microenvironment are sufficient to trigger this change in cancer cells and thereby initiate metastasis. Going partway through the EMT also makes cancer cells resistant to many treatments, including immunotherapies. Weinberg lab researchers have shown that if only 10% of the cancer cells in a tumor do so, they can protect the entire tumor against immunotherapy. They do this by releasing signals into the tumor microenvironment that keep out immune cells that could otherwise attack the tumor and let in immune cells that could help it.
As cancer cells spread throughout the body, they encounter new tissue environments to which they may be less well adapted. As a consequence, many such cancer cells end up in a dormant, non-proliferative state – often triggered by signals from their new microenvironment. However, if conditions in the surrounding tissue microenvironment change, this may lead to the awakening of these previously dormant cells. The ability of cancer cells to reawaken after long dormancy appears to explain how cancers may recur years after the original primary tumor has been eliminated.
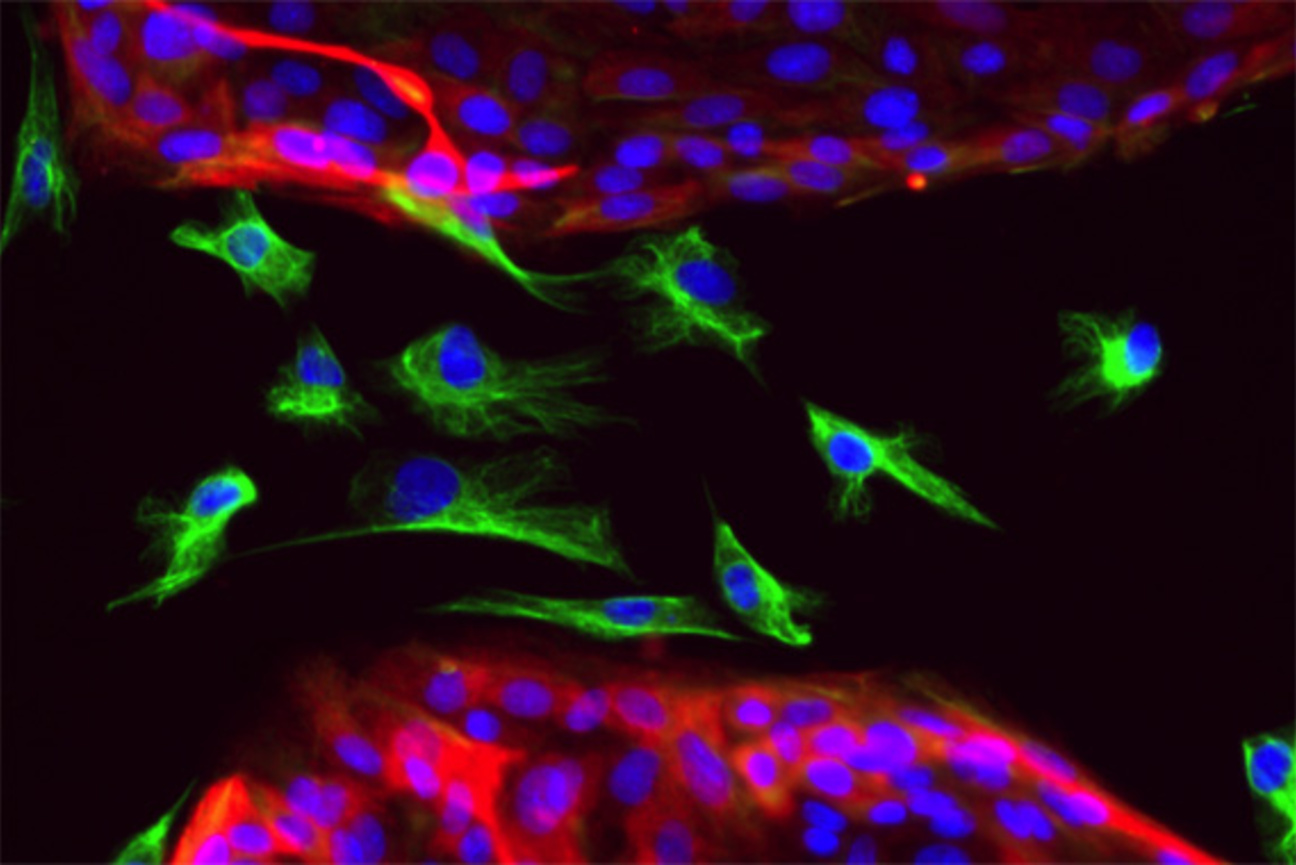
During an epithelial-to-mesenchymal transition (EMT), epithelial cells acquire the traits of mesenchymal cells. Unlike the tightly-packed epithelial cells (red with blue nuclei) that stick to one another, mesenchymal cells (green with blue nuclei) are loose and free to move around a tissue. The attributes of mesenchymal cells are beneficial during development, but when hijacked by cancer cells, confer the ability to migrate to distant sites.
Christina Scheel/Whitehead Institute
More specifically, researchers in Weinberg’s lab have studied how inflammation in the tissue microenvironment surrounding cancer cells can lead to their awakening. Moreover, cancer patients who undergo surgery to remove a primary tumor may experience inflammation at the site of post-surgical wound healing. Weinberg’s research in mice, along with others’ studies of breast cancer patients, suggests that patients who take an anti-inflammatory drug—such as an over-the-counter NSAID—after their surgeries are less likely to experience new metastases. This is one example of how cancer metastasis can potentially be prevented by changing the state of the cancer cells’ environment without having to directly target the cancer at all.
Cancer cells thrive by manipulating their environments to work for them; now researchers are developing therapies that can make their environments work against them. As some researchers work to develop these therapies, it will also be important for basic researchers, like those at Whitehead Institute, to keep exploring how cancer cells interact with their environments and what the consequences of those interactions are. When researchers understand all of these interactions on a fundamental, mechanistic level, they will know what makes a cancer thrive or fail to thrive within a given tissue microenvironment, and so understand how to best change the microenvironment within a cancer patient in order to ensure that their cancer fails to thrive.
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


