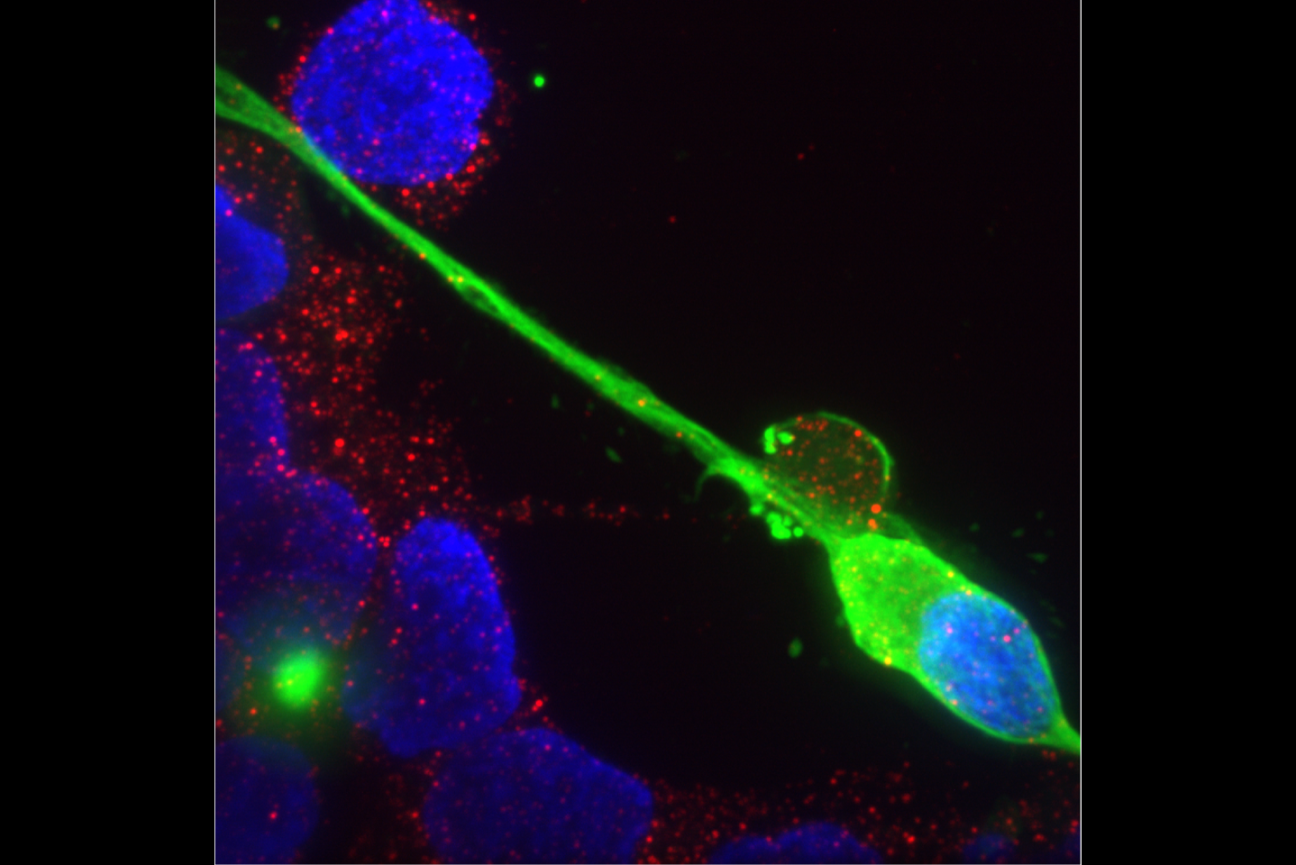
Sensory neurons infected with SARS-Cov-2, the virus that causes Covid-19.
Figure 2, Flamier, A., Bisht, P., Richards, A., Tomasello, D.L, Jaenisch, R., Human iPS cell-derived sensory neurons can be infected by SARS-CoV-2, ISCIENCE (2023), doi: https://doi.org/10.1016/j.isci.2023.107690
SARS-Cov-2, the virus behind Covid-19, can infect sensory neurons
When Covid-19 began spreading in 2020, some of its most common symptoms affected the peripheral nervous system, the network of nerves that enables communication between the brain and the rest of the body. Many people reported losing their senses of smell and taste, which both rely on sensory neurons in the peripheral nervous system. In the years since, a significant number of people who have contracted Covid-19 have reported developing some sort of neuropathy, meaning a dysfunction of the peripheral nerves that typically causes numbness, weakness, and/or pain. Sometimes, these symptoms persist and become long-term health issues.
Researchers puzzled over how SARS-CoV-2, the virus responsible for Covid-19, could be causing these symptoms, as early evidence suggested that the virus could not infect neurons or only infected them rarely. Viral diseases can impact cells without directly infecting them, such as through damage caused by the body’s immune response to the virus. However, the timing of when most people developed neuropathies did not align with the peak of the immune response to Covid-19.
As the number of people affected by peripheral nervous system symptoms of Covid-19 grew, and the question of whether SARS-CoV-2 could infect neurons remained unresolved, Whitehead Institute Founding Member Rudolf Jaenisch and then-postdoc in his lab Anthony Flamier, now a research assistant professor at CHU Sainte-Justine, wanted to find a definitive answer. Their research, published in the journal iScience on September 15, shows that SARS-CoV-2 can infect sensory neurons, leading to changes in the cells’ gene expression. These findings may help to explain how the virus causes symptoms in the peripheral nervous system, laying a foundation for researchers to develop treatments.
“There is clearly a clinical effect of SARS-CoV-2 infection on sensory neurons, such as on smell and taste, and we did not know what might be the cause,” says Jaenisch, who is also a professor of biology at the Massachusetts Institute of Technology. “Knowing that the virus can infect and probably alter the function of the cells gives us a hint of what the cause might be.”
Flamier and colleagues differentiated induced pluripotent stem cells into human sensory neurons in the lab. They confirmed that the cells had differentiated into sensory neurons by checking that they expressed key genes specific to that cell type. Next, the researchers tested to see if the sensory neurons expressed ACE2, the gene encoding the protein that SARS-CoV-2 uses to gain entry into human cells. They found that the sensory neurons did express ACE2, at a level comparable to other cell types known to be infected by the virus—a first indication that sensory neurons likely could be infected.
The researchers then exposed the sensory neurons to three strains of SARS-CoV-2, the original WA1/2020 strain, and the Delta and Omicron strains that caused surges in Covid-19 infection. The researchers sequenced RNA from the sensory neurons and found that all three strains of the virus infected some portion of the sensory neurons. Omicron infected the lowest percentage of cells within the same timeframe, suggesting that it is slower than the other strains to infect sensory neurons. This finding could help to explain why Omicron infection leads to lower rates of loss of taste and smell than the previous strains did, but further research would be needed to prove a connection.
Next, the researchers looked at how gene expression changed in infected sensory neurons. Each viral strain affected the expression of different genes, but overall, the most impacted genes were those involved in immune response. Additionally, genes involved in RNA synthesis and processing showed reduced expression. These are genes whose function the virus may co-opt to its own benefit, and so their reduced expression could reflect the cell attempting to defend itself against the virus.
Although the virus was able to infect sensory neurons, the researchers found that it could not make new copies of itself inside them. When SARS-CoV-2 infects other cell types, such as lung cells, it creates lots of new copies of itself, ultimately killing the host cell and allowing the virus to keep spreading and infecting more cells. However, the infected sensory neurons neither died nor emitted any copies of the virus. Flamier is now working on figuring out how the cells achieve this.
“The next step will be to understand how sensory neurons survive the virus, and why the virus cannot reproduce in these cells,” Flamier says. “If we know that, it will help us understand the biology of Covid-associated neuropathies, and it could possibly even be useful for treating other cell types that do die when infected by the virus, like lung cells.”
The better researchers understand the interactions between the virus and sensory neurons, the more insight they will have into the mechanisms by which SARS-CoV-2 affects human cells, and the mechanisms by which human cells attempt to defend themselves from the virus. These insights could pave the way for new Covid-19 therapies that would improve the quality of life of many millions of people living with lingering neuropathies after Covid-19.
Flamier, A., Bisht, P., Richards, A., Tomasello, D.L, Jaenisch, R., Human iPS cell-derived sensory neurons can be infected by SARS-CoV-2, ISCIENCE (2023), DOI: https://doi.org/10.1016/j.isci.2023.107690.
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


