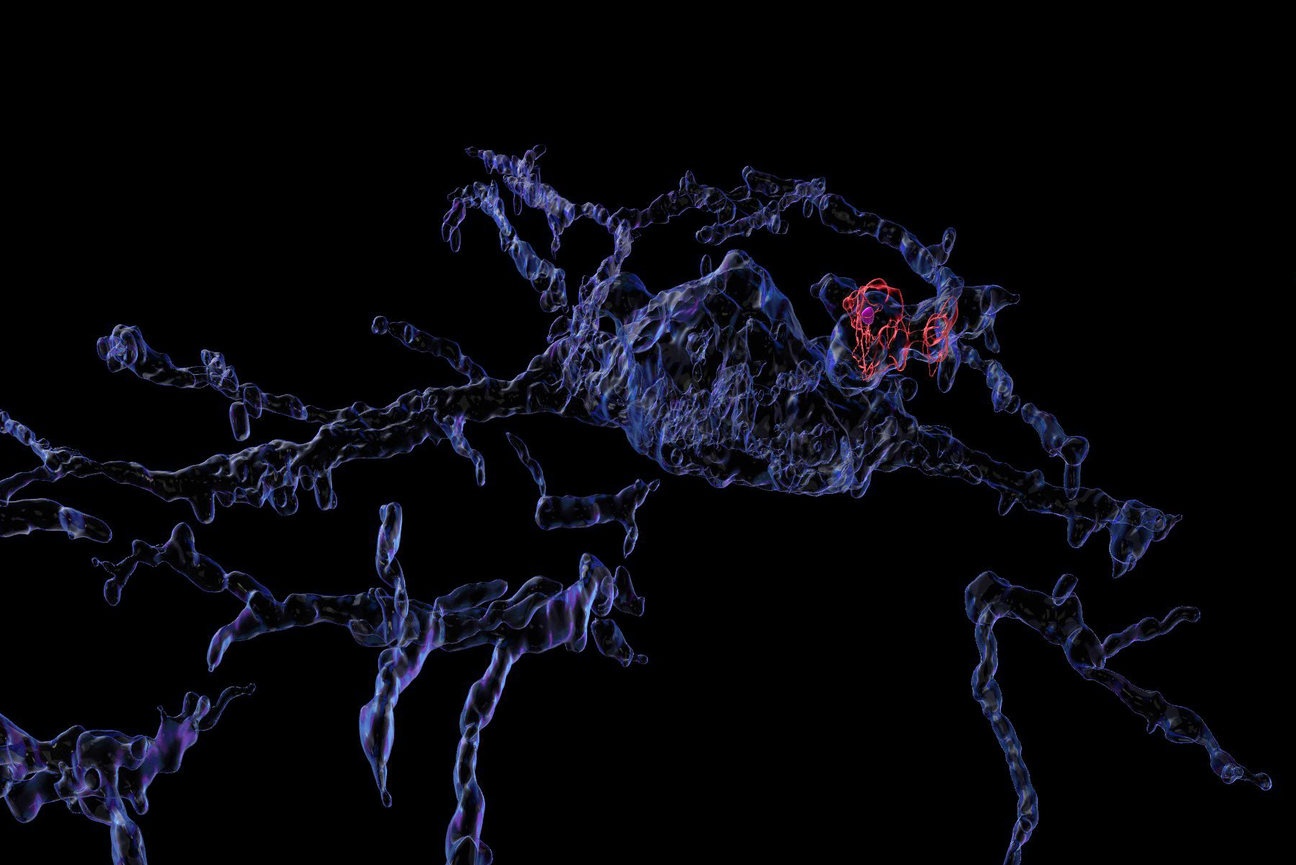
Volume-rendered confocal image depicting a microglia (blue) and its phagosome (red) containing phagocytosed synaptic material (magenta).
Emile Wogram
New approach enables a closer look at brain cell organelle
Microglia are the immune system’s front-line enforcers in the brain. They are cells that patrol the brain and destroy anything harmful that they encounter, from invading bacteria to cellular debris. They also remove plaques and prune dysfunctional synapses between neurons. Microglia eliminate their targets by eating them: they envelope material and seal it in bubble-like organelles called phagosomes. A phagosome can then fuse with other organelles that break down its contents.
Microglial phagosomes play important roles in brain development, brain function and a plethora of brain diseases, including neurodegeneration and brain cancer. Therefore, understanding microglial phagosome biology could help to develop new therapies for currently untreatable brain diseases. However, microglia and their organelles have been difficult to study because existing stem cell and animal models insufficiently resemble microglia in the human brain, and because microglia, as vigilant immune patrollers, react to even subtle stimuli and so experimental conditions can trigger changes in the cells that confound analyses.
To overcome those issues, Whitehead Institute Founding Member Rudolf Jaenisch, also a professor of biology at the Massachusetts Institute of Technology; University of Freiburg Professor of Neuropathology Marco Prinz; and University of Freiburg neuropathologist Emile Wogram, who began this project as a postdoctoral researcher in Jaenisch’s lab, have developed a method to isolate and analyze microglia phagosomes in a rapid, gentle, and unbiased fashion.
In research shared in the journal Immunity on August 15, the researchers describe how they can isolate and profile phagosomes from stem cell-derived microglia and fresh human brain tissue. They also share new insights into phagosome biology in the human brain, regarding synaptic pruning and generation of NAD+, a broadly used molecule in the brain, by microglia.
The method that the researchers developed to isolate phagosomes from cells uses immunoprecipitation, in which antibodies latch on to a specific target protein on an organelle’s surface. When the antibodies are collected, they pull the organelles with them. This technique avoids many chemical perturbations that might alter the microglial profile. Sometimes researchers genetically engineer a target for the antibodies, but in order to isolate phagosomes from human brain tissue, Wogram had to find a naturally expressed target. Eventually, he and colleagues found one: the protein CD68.
The researchers first isolated phagosomes from stem cell-derived microglia. They co-cultured the microglia with other brain cell types to create a more brain-like environment, which led to a better match between brain and stem cell-derived microglia gene expression. They triggered some of the microglia to enter an inflammatory or disease-like state to see how that affected the phagosomes. Additionally, Wogram collaborated with the neurosurgery department at the University of Freiburg to get access to brain tissues immediately after their removal during surgery. He isolated phagosomes from brain tissue within a half hour of its removal, allowing him to profile the organelles before their contents could change much.
The profiles that the researchers built included what proteins and metabolites the phagosomes contained, and the whole-cell gene expression profile. The profiles differed significantly between sets of phagosomes, but the researchers identified a core of consistent proteins, including many known and also some unknown phagosome proteins. The results showed that phagosomes contain sensitive signaling molecules that allow them to react quickly to even subtle environmental stimuli.
Additionally, the protein contents of the co-cultured microglia provided strong evidence that when microglia prune synapses, they predominantly prune the side that sends a signal and not the side that receives one. This insight could be useful for understanding how microglia interact with synapses in health and disease.
The researchers also gained insights into a key metabolic pathway that occurs inside of microglia. In excess, the molecule quinolinic acid can be toxic to neurons; it is implicated as involved in many neurodegenerative diseases. However, cells can use quinolinic acid to make NAD+, a molecule broadly used to carry out essential cellular functions. Microglia are the only brain cells that generate NAD+. Wogram and colleagues found that key steps in this process occur in phagosomes. Phagosomes are therefore necessary both for removing excess quinolinic acid to prevent toxicity and for helping to generate NAD+ in the brain.
Finally, Wogram used brain tissues to compare phagosomes from within a tumor to those in the surrounding healthy tissue. The phagosomes in the tumor contained excess quinolinic acid. Although follow-up studies would be needed to confirm the results, these findings are consistent with research that suggests cancer cells use quinolinic acid to fuel their growth.
Collectively, these findings illuminate aspects of phagosome biology and the roles that phagosomes may play in normal brain development and maintenance, as well as in cancer and neurodegeneration. The researchers also anticipate that their method could prove useful for profiling other organelles, especially when the organelles need to be rapidly isolated from human tissue.
Wogram et al. "Rapid phagosome isolation enables unbiased multiomic analysis of human microglia phagosomes." Immunity, August 15, 2024. DOI: 10.1016/j.immuni.2024.07.019
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


