
Jennifer Cook-Chrysos/Whitehead Institute
Conducting science during a pandemic
This story is part of our 2020 Annual Report. Download the full report here.
In March 2020, a team of Whitehead Institute scientists and administrators planned and implemented something few had ever imagined: quickly ramping down the Institute’s scientific operations to help limit spread of the novel coronavirus in the Boston region. By early summer — when the COVID-19 curve had flattened and Institute labs began, slowly, moving toward fuller operation — the work of every Institute scientific and technical staff member had been affected.
It is an understatement to say that the process of ramping down and then restarting research operations was multifaceted, detailed, challenging, and frustrating. “Scores of researchers had projects slowed in significant ways, and some had projects stopped in their tracks,” said Institute Member and former Director David Page, who led the pandemic-response processes from March through June 30, when Ruth Lehmann succeeded him as director. “The true impact on scientific discovery — and on the professional growth of our postdocs and early career scientists — will only become clear in coming years. But it certainly feels like we, collectively, have lost momentum on many fronts.”
Yet the Whitehead Institute scientific community was able to keep an array of scientific projects moving forward through the difficult spring and early summer months. A special team worked with Page to review projects critical to each lab and, on a case-by-case basis, approved the continuation of specific work. For example, many projects that involved specially developed animal models continued, albeit at a slower pace due to limitations on the number of people who could occupy the lab at any one time. So, too, did work on infectious disease and the Institute’s many projects utilizing human stem cells.
Perhaps the most significant ongoing work involved projects where Institute researchers applied their specialized tools and methods to delve deeply into how SARS-COV2 functions — and thereby lay the groundwork for potential COVID-19 therapeutics. “This is an important example of the broad application of the foundational science we do,” said Ruth Lehmann. “Our scientists are directly applying their knowledge, expertise, and technical capacities to the virus.” Five examples of these projects are featured below.
Silvi Rouskin in the lab.
Gretchen Ertl/Whitehead Institute
Silvi Rouskin
For the past several years, Whitehead Fellow Silvi Rouskin’s lab has been developing methods to determine the conformation of RNA viruses such as HIV. In February, as SARS-COV2 — an RNA virus — spread around the world, Rouskin recognized that research methods created by her group could be directly applicable to the long-term development of COVID-19 treatments.
“No one knew what the RNA structure of SARS-COV2 was,” she said. “That's important because understanding the RNA’s shape is critical for designing RNA-based therapeutics.” Her group undertook to define that shape. When the Institute began ramping down, the lab was already deep into the project. Given its progress and relevance, Rouskin received permission to continue the work. “We were self-contained and self-sufficient; we had all the equipment we needed in our lab.” Two months later, they had mapped the virus’s RNA structure and made the preprint publication available to the scientific community through the open access repository bioRxiv. Rouskin is now working with collaborators to screen small-molecule drugs capable of incapacitating the viral RNA.
Gretchen Ertl
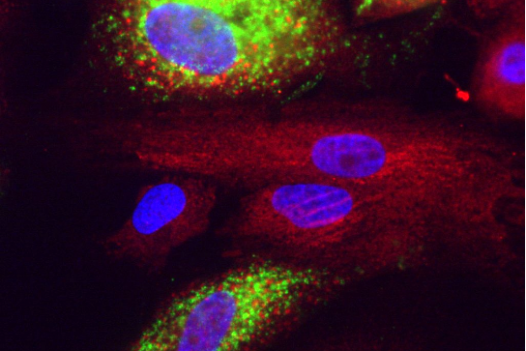
Covid in liver cells. Green is dsRNA, red is calreticulin, blue is nuclei stained with DAPI.
Courtesy of Rudolf Jaenisch
Rudolf Jaenisch
When COVID-19 emerged, Founding Member Rudolf Jaenisch recalled, “We decided to use our expertise in stem cells to investigate which cells in the body are directly and indirectly affected by the coronavirus and how different cell types respond.” In one project, Jaenisch’s group has been inducing embryonic stem cells to differentiate into every type of cell in the body, then screening them to see how they respond to coronavirus infection. Alexsia Richards — a postdoc in Jaenisch’s lab who is the only person at MIT with safety clearance to work with the virus — transfers the newly differentiated cells to a biosecure facility at the Ragon Institute of MGH, MIT and Harvard; there she infects the cells with the coronavirus and analyzes the results. In a second project, the Jaenisch lab — collaborating with a group at Harvard University that has lung tissue samples from COVID-19 patients — has created lung organoids that comprise all the different cell types of the lung in a 3-D system. “We have found that certain types are infectable and others are not,” Jaenisch explained, “and we are trying to understand how to block the infection process.”
Kipp Weiskopf
Gretchen Ertl
Kipp Weiskopf
Whitehead Fellow Kipp Weiskopf’s lab studies interactions between cancer cells and the immune system and is pursuing methods to prompt the immune cells called macrophages to fight the disease. When the pandemic hit, he realized that efforts to promote macrophage activity might be repurposed to identify treatments for severe cases of COVID-19. The connection? In some patients, hyperactive macrophages trigger an immune reaction called a cytokine storm, which can cause more damage to organs than the virus itself would.
“We saw an opportunity to use the tools and models our lab had previously created toward a new goal: investigating whether and how drugs that inhibit macrophages may prevent the cytokine storm,” Weiskopf explained. His lab received special approval to pursue this work during the spring shutdown. Now they are collaborating with the Broad Institute to screen thousands of existing drugs for their ability to inhibit macrophage activation. “It’s a prime example of how research in one area of fundamental biology can have implications for many different diseases,” he noted, “and over the coming year, we will begin studying potential drug candidates’ effects in animal models and in vitro.”

Jonathan Weissman
New Institute Member Jonathan Weissman has pursued two COVID-related projects. The first involves his gene-silencing technique, CRISPR-OFF, which, rather than cutting DNA with CRISPR’s molecular scissors, allows researchers to methylate the gene, rendering it inactive but intact. “We are using this, along with technologies that Rudolf Jaenisch pioneered, to try to develop a system that could shut off the lung epithelial cell genes required for coronavirus to infect those cells,” Weissman explained. “This could create a sort of immunity that protects against both SARS-COV2 and future SARS-like coronaviruses.” His lab’s second COVID-relevant project has involved a closer look at the specific components of human cells that viruses hijack to replicate their genetic material. Building on other researchers’ identification of proteins that SARS-COV2 needs to reproduce, Weissman has been studying where in the viral life cycle those proteins are acting. In the long run, he said, “These projects could lead to inhaled RNA-based treatments that go into the respiratory tract and use CRISPR-OFF to shut off key host factors used by the virus.”
Richard Young
Gretchen Ertl
Richard Young
Institute Member Richard Young is a pioneer in the emerging field of bimolecular condensates — and in studying condensates’ potential role in COVID-19 therapeutics. These membraneless droplets concentrate certain proteins, effectively providing shelter from the broader chaos of the cell and enabling complex functions to take place. Condensates help the cell transcribe its genetic material, produce ribosomes, and splice RNA. “Condensates provide an important new lens on cell biology,” Young observed, “and hold the potential for both explaining many mysteries and raising many interesting new questions.” Young has been applying his knowledge of these cellular droplets to better understand how viral infection takes place. Currently his lab is investigating how condensates may contribute to the virus’s ability to replicate itself in human cells. And he and his collaborators — research leaders in biology, chemistry, physics, and artificial intelligence — are using condensates as the basis for a whole new paradigm for drug discovery. “We see increasing evidence that condensates play a significant role in distribution of a therapeutic within a cell and, therefore, in its effectiveness and efficiency,” Young explained. “And we have been studying the role that condensates may play in the delivery of drugs that interfere with the coronavirus’s function.”
Course faculty, from left to right: Teaching Assistant Lena Afeyan, Professor Facundo Batista, and Professor Richard Young.
Screenshots courtesy of MIT Biology, graphic by Ceal Capistrano
Teaching About COVID-19
In parallel with his scientific work on the SARS-COV2 virus, Institute Member Richard Young partnered in developing and leading a special MIT Department of Biology course entitled “SARS-CoV-2, COVID-19 and the Pandemic.” The course addressed the pandemic from an array of perspectives, including virology, immunology, epidemiology, public health, and clinical care. It was presented by a “Who’s Who” of biomedical experts including Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases; Whitehead Institute Founding Director David Baltimore; Bruce Walker, director of the Ragon Institute of MGH, MIT, and Harvard; Arlene Sharpe, chair of the Department of Immunology at Harvard Medical School; and others at the forefront of COVID-19 research and treatment. Co-developed and co-led by Young’s MIT faculty colleague Facundo Batista, who is associate director of Ragon Institute and an expert on immunology and infectious disease, the course was presented through a weekly livestream during MIT’s fall semester. Then each lecture was made available to the general public on YouTube. It is estimated that the lectures collectively had tens of thousands of views.
Moving Forward
Taking in a tumultuous 2020, Lehmann observed: “Last spring, everyone was caught off guard by COVID-19. David Page and his team did a great job in making and implementing the decision to substantially ramp down Institute operations to help contain the spread of the novel coronavirus, and then in beginning the process of ramping up again during the summer.
“But looking forward, we need to anticipate facing this kind of situation again and consider how best to address a dual imperative: protecting public health while continuing to advance our research and training programs. We must keep science moving ahead. And we must find ways to prevent monthslong setbacks in our students’ and postdocs’ careers.”
If there is any silver lining to the pandemic’s effect on Whitehead Institute, Lehmann said, “It’s that we have seen our resilience tested and proven. We’ve demonstrated our collective capacities for flexibility, creativity, and nimbleness. And we have shown, again, how relevant is the work we do and how useful are the new methods and tools our researchers are continually developing.”
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


