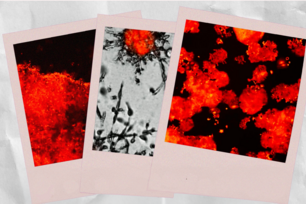
According to Whitehead Institute researchers, protein production or translation is tightly coupled to a highly conserved stress response—the heat shock response and its primary regulator, heat shock factor 1 (HSF1)—that cancer cells rely on for survival and proliferation. One compound that is particularly effective at disrupting translation and HSF1 activity is Rohinitib (RHT). In cancer, a state marked by increased protein translation and biomass expansion, HSF1 is activated and binds to numerous genes throughout the genome, as seen in the heat map of HSF1 ChIP-Seq read density in M0-91acute myeloid leukemia cells (DMSO, far left). When added to these cells, RHT reverses HSF1's cancer-associated activation (RHT (100nM), left). Cancer cells, including the M0-91 cells, have increased glucose uptake due to their abnormal metabolism. Marking these cells with a green dye to indicate their glucose uptake shows that RHT significantly reduces M0-91cells' glucose uptake (RHT, far right) compared to untreated cells (Control, right).
Courtesy of AAAS
Thwarting protein production slows cancer cells’ malignant march
CAMBRIDGE, Mass. – Protein production or translation is tightly coupled to a highly conserved stress response that cancer cells rely on for survival and proliferation, according to Whitehead Institute researchers. In mouse models of cancer, targeted therapeutic inhibition of translation disrupts this survival response, dramatically slowing tumor growth and potentially rendering drug-resistant tumors vulnerable to other therapies.
From yeast to worms to humans, this stress response and its primary regulator, heat shock factor 1 (HSF1), help normal cells adapt to harsh environments, including the presence of heavy metals, high salt concentrations, low oxygen levels, and of course increased temperatures.
"In a perverted twist of fate, cancer cells take advantage of this incredibly ancient survival strategy—the heat shock response—to help them survive despite the best efforts of our own natural defenses, and sophisticated therapeutics, to kill them,” says Whitehead Member Susan Lindquist. “And trumping all that, we find it not only helps them survive, it helps them thrive!"
Across tumor and cancer types, cancer cells rely on the heat shock response and HSF1 to support the production of vast quantities of proteins and the high-energy demands needed to propel malignancy. Accordingly, researchers have envisioned HSF1 as a potential therapeutic target, but such transcriptional regulators have been notoriously difficult to target. However, by determining that protein translation is intimately connected to HSF1 activity, Whitehead scientists may have identified an approach to controlling cancer cells’ overactive heat shock response. Their work is described in this week’s issue of the journal Science.
“The genetic screens that we conducted in collaboration with the Broad Institute and the drug screens that were conducted by Sandro Santagata (Lindquist lab postdoctoral researcher) all pointed to this connection—that the process of protein production signals to HSF1,” says Marc Mendillo, a postdoctoral researcher in Lindquist’s lab and a coauthor of the Science paper with Santagata. “And this link may explain the HSF1 activation we have observed across an extraordinarily broad range of human cancers.”
Santagata’s screens identified one compound that was particularly effective at disrupting translation and HSF1 activity. Collaborators at Boston University synthesized an analog of this compound, called Rohinitib (RHT), that is even more efficacious. Normal cells are relatively resistant to RHT and seem to be little affected by it. However, cells from a wide spectrum of cancers are sensitive to it—RHT added to cancer cells in vitro normalizes their metabolism, including the increased glucose uptake characteristic of such cells, and even kills them. Blood cancer lines are highly sensitive to RHT and show the most dramatic effects. In mice implanted with human myeloid leukemia tumors, RHT greatly inhibited the tumors’ growth and suppressed glucose uptake, similar to the effects seen in vitro.
“I think we’ve found a very simple but elegant biological principle here, which makes sense,” says Santagata. “Systems in the cell that need to work together—such as protein translation and the heat shock response—actually are linked together. We found that link using small molecules, which means that we now have the tools in hand to suppress what cancer has coopted. We can use those chemicals to thwart the cancer cells’ ability to harness the properties of HSF1.
Such chemicals may be enough to knock cancer cells off balance, but the final coup de grace may need to come from other therapeutics.
“You probably want to have these kinds of effects in the context of other therapeutic interventions,” says Luke Whitesell, an oncologist and senior research scientist in the Lindquist lab. “If you were to compromise the altered physiology of tumors with something like RHT, the cancer cells are going to be less able to tolerate other therapeutic insults, and that probably would give you more effective therapies. But we don’t know what the best combinations are going to be yet.”
This work was supported by the Johnson & Johnson Focused Funding Program, the Marble Fund, the National Institutes of Health (NIH grants R01 CA175744-01, R03 MH086465-01, R03 DA027713-01, 5U54HG006093, R01 GM073855, K08NS064168), American Cancer Society New England Division –SpinOdyssey (PF-09-253-01-DMC), the Brain Science Foundation, the American Brain Tumor Association, the Beez Foundation, the V Foundation, and the Jared Branfman Sunflowers for Life Fund.
* * *
Susan Lindquist’s primary affiliation is with Whitehead Institute for Biomedical Research, where her laboratory is located and all her research is conducted. She is also a Howard Hughes Medical Institute investigator and a professor of biology at Massachusetts Institute of Technology.
* * *
Santagata, S. et al. “Tight Coordination of Protein Translation and HSF1 Activation Supports the Anabolic Malignant State." Science, July 18, 2013
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu