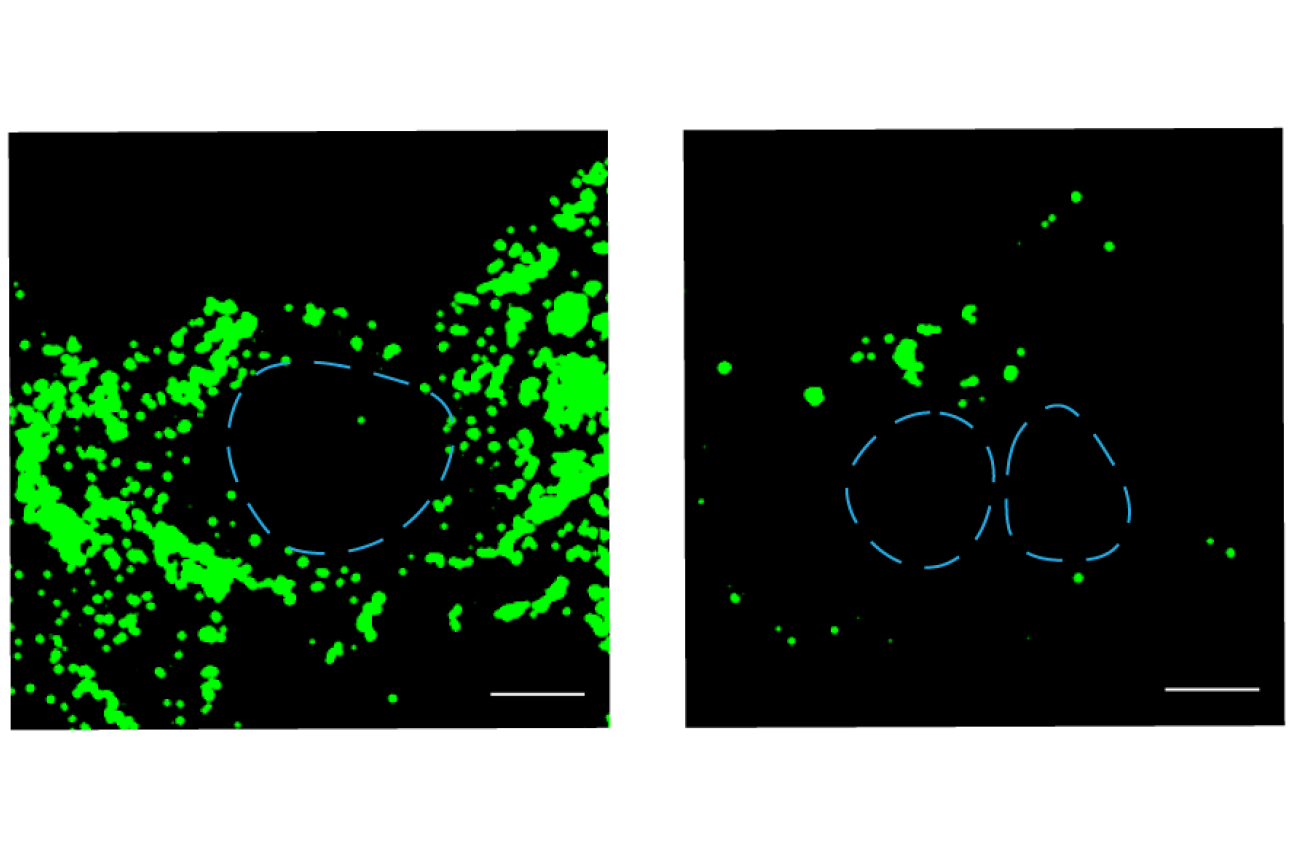
Insulin receptors cluster differently in cells from people with diabetes (right) than in healthy cells (left).
Excerpted from Figure 1, Dall’Agnese, A., Platt, J.M., Zheng, M.M. et al. The dynamic clustering of insulin receptor underlies its signaling and is disrupted in insulin resistance. Nat Commun 13, 7522 (2022). https://doi.org/10.1038/s41467-022-35176-7
A new understanding of diabetes
Type 2 diabetes affects hundreds of million people around the world, is responsible for many medical complications and millions of deaths each year, and its prevalence is on the rise. Due to the growing strain of diabetes on those who suffer from it as well as on our public health systems, significant time and energy has gone into searching for breakthroughs in our understanding of the disease that could lead to better treatment and prevention. Researchers in Whitehead Institute Member Richard Young and Whitehead Institute Founding Member Rudolf Jaenisch’s labs have been hunting for such a breakthrough with support from Novo Nordisk, a healthcare company dedicated to defeating diabetes. Recently, that work bore fruit.
Research led by Young lab postdocs Alessandra Dall’Agnese and Jesse Platt and research scientist Tony Lee has uncovered a molecular mechanism underlying type 2 diabetes. The new finding, made in collaboration with researchers from the labs of Jaenisch and Massachusetts Institute of Technology (MIT) Professors Linda Griffith and Ibrahim Cissé, reveals that insulin receptors, the signaling molecules that sense insulin, normally function by clustering together in cells and that this clustering is defective in insulin resistance, the basis of type 2 diabetes. Their discovery also helps explain a mystery about how therapeutic treatment by metformin, the frontline drug for diabetes, works in patients. The researchers hope that these insights, published in Nature Communications on December 6, will bring about a better understanding of diabetes at a molecular level and lead to the development of new therapies.
“There are over one billion people that have insulin resistance, and this predisposes them to type two diabetes, cardiovascular complications, and more—and the number of people affected worldwide keeps increasing,” Dall’Agnese says. “My goal is that people can use this new finding to develop drugs to treat patients with insulin resistance at the stage before it has led to severe health complications.”
When insulin can’t do its job
Type 2 diabetes is one of several diseases linked to insulin resistance, in which cells become less receptive to insulin. After a person eats, the level of sugar in their blood increases, and the body releases insulin into the blood in response. Insulin receptors in cells throughout the body sense this insulin and then prompt cells to take in sugar to use as fuel. Signaling from insulin receptors also triggers other important metabolic processes. However, cells that develop insulin resistance cannot properly react to the presence of insulin. This means too much sugar stays in the blood instead of entering cells, leading to a variety of symptoms.
Dall’Agnese and Platt discovered that insulin receptors cluster together in cells, forming droplets called condensates, and that these droplets are dysregulated in insulin resistant cells. Condensates form when molecules cling together in big groups, creating a droplet that separates out from the main liquid of the cell like oil suspended in water. These condensates concentrate additional components needed for insulin signaling and are highly dynamic: they form, fuse, deform, and dissolve often on the scale of seconds. The researchers believe that the dynamic nature of the condensates enables all of the molecules within to rapidly interact, making the insulin receptors’ activity more efficient.
A change in condensate activity
When the researchers looked at healthy liver and fat cells, they found that insulin receptors formed condensates throughout the cell. Increases in insulin led more insulin receptors to join condensates. However, when the researchers looked at liver cells from people with type 2 diabetes, as well as liver and fat cells engineered to mimic the disease, they found that insulin receptors’ ability to join condensates was impaired in a number of ways. In these cells, insulin receptors formed fewer condensates, each condensate contained fewer insulin receptors, and the condensates were less dynamic.
“The condensate droplets behaved more like honey than water. This prevents the molecules from moving around quickly, so each insulin receptor will see fewer of the molecules that it is supposed to interact with,” says Young, who is also a professor of biology at MIT.
Correspondingly, the researchers found that insulin receptors were less active in these condensates, as measured by a decrease in one of the molecules their activity creates. Additionally, the researchers observed that whereas increases in blood insulin led more insulin receptors to join condensates in healthy, insulin sensitive cells, it did not do so in the insulin resistant—or diabetic—cells.
The researchers then looked at what happened to the insulin receptor condensates in people with type 2 diabetes who were treated with metformin. Looking at patient cells and cells grown in the lab, they found that metformin restored much—though not all—of the condensates’ presence and dynamics, and much of the normal activity of insulin receptor.
Taken together, these findings suggest that one mechanism by which insulin resistance can affect cells—specifically liver and fat cells, two key tissues in diabetes—is by dysregulating insulin receptors’ ability to join and function in condensates in response to rises in insulin levels. Likewise, one way in which metformin may work is by restoring normal condensate activity.
Looking forward to helping patients
This discovery opens up a new area of drug development for diabetes: drugs that will restore normal condensate activity for insulin receptors. Such a drug could be used as an alternative for people who cannot tolerate metformin or who have lost sensitivity to it, and could potentially be designed to avoid some of the negative side effects of metformin. The researchers see a number of valuable uses for a condensate-targeting drug: it could be given to prediabetic patients to prevent the development of more severe disease. It could benefit people who have other diseases linked to insulin resistance, such as fatty liver disease, polycystic ovary syndrome (PCOS), and metabolic syndrome. Furthermore, it could be used as a jumping off point to design drugs for other diseases in which condensate dysregulation is implicated, ranging from cancer to heart disease to neurodegeneration.
“I see a lot of patients in the clinic who have insulin resistance,” says Platt, who is also a hepatologist at Massachusetts General Hospital. “For my entire clinical training, it’s been mysterious how insulin resistance comes about and leads to disease. Discovering this mechanism could be a chance to help millions of people. I’m excited about what this could mean for my patients, and I’m also hopeful that this could be an opportunity to help people with many different diseases.”
Citation: Dall’Agnese, A., Platt, J.M., Zheng, M.M. et al. The dynamic clustering of insulin receptor underlies its signaling and is disrupted in insulin resistance. Nat Commun 13, 7522 (2022). https://doi.org/10.1038/s41467-022-35176-7
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu

