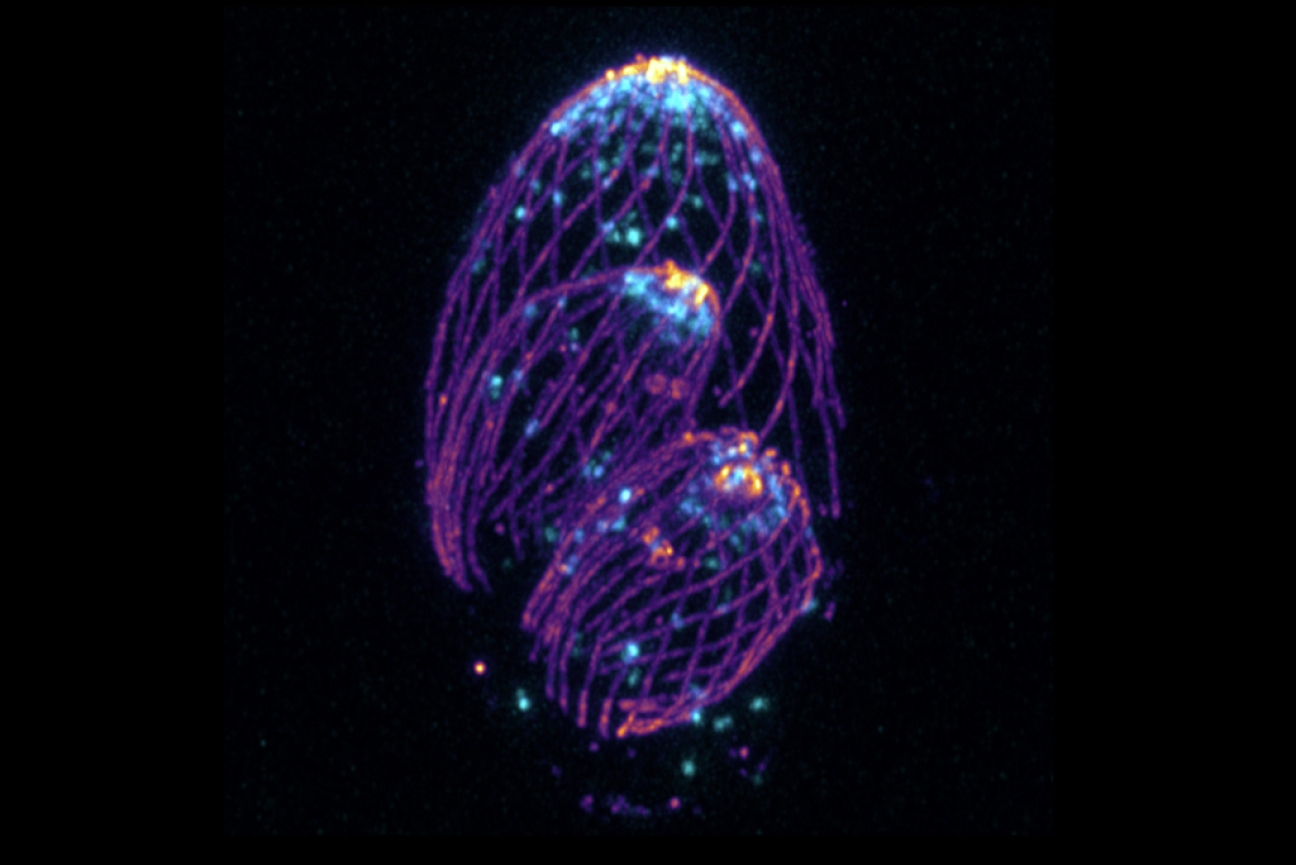
A T. gondii parasite undergoing cell division. Micronemes, packages that carry molecules the parasite needs to travel to and invade new cells, are labeled in blue and microtubules, the cytoskeletal highways the micronemes move along, are labeled in purple.
Alex Chan/ Whitehead Institute
How parasites get their HOOKs into human cells
Apicomplexan parasites are single-celled organisms that infect millions of people a year around the world. Different species of apicomplexans are responsible for spreading malaria, cryptosporidiosis (a severe diarrheal disease that affects young children), and other serious illnesses. Whitehead Institute Member Sebastian Lourido studies apicomplexan biology using the species Toxoplasma gondii (T. gondii), which causes toxoplasmosis, a disease that can be deadly in people with compromised immune systems. Uncovering new aspects of the parasites’ biology could lead to therapies to eliminate them or prevent disease symptoms.
Active T. gondii parasites transition between two modes: replicating inside of one host cell, and spreading to new cells. The transition between these modes happens very quickly: parasites will be busy replicating and then will rapidly exit the host cell and travel to new cells to invade them. Once inside a new cell, the parasites can switch back to reproducing or enter into a chronic stage—a hardy, inert form that can hide from the host’s immune system indefinitely.
Researchers knew that T. gondii starts its transition from the reproductive to the spreading mode with a calcium signal within the parasite, but they did not know much about the chain of cellular events between that first signal and the actual switch in modes. In research published in the journal eLife on November 7, Lourido, former graduate student in his lab Alex Chan, and colleagues shed light on what happens inside of the parasite cell to enable this transition.
Piecing together the chain of events
Calcium is an important signal that many cells use to trigger the quick release of different molecules, such as neurotransmitters from neurons and insulin from pancreatic cells in humans. In T. gondii, calcium is the signal that triggers release of molecules parasites need to exit host cells, travel, and invade. These include molecules to make the host cell membrane permeable and sticky molecules that the parasite uses to attach to and glide along host tissue, enabling it to move in the environment outside of the host cell. These molecules are packaged in small membrane-bound containers called micronemes, hundreds of which are scattered throughout the front third of the parasite cell.
The calcium signal begins a chain of signaling, like a game of telephone, that ultimately triggers the parasite cell to release the contents of these microneme packages. The cell does this by attaching the packages to a cellular conveyor belt system that runs along microtubules, part of the cytoskeleton. This whizzes them to the very front of the parasite, where their contents can be released outside of the parasite through a process called exocytosis, in which the membrane of the package fuses with the membrane of the parasite cell.
When T. gondii parasites get the signal to exit the host cell and travel, the micronemes (in yellow) get quickly shuttled to the very tip of the parasite cell.
Alex Chan/ Whitehead Institute
“Parasites need to control how and when they interact with host cells strategically, in order to survive in a hostile environment,” says Lourido, who is also an associate professor of biology at the Massachusetts Institute of Technology. “They need to release the contents of micronemes precisely at the moment when they’re undertaking the transition from replication to movement, and so this transition happens incredibly quickly. How they achieve that, at a cellular and molecular level, has remained quite mysterious.”
What Lourido and Chan wanted to understand was what happens between the calcium signal and the release of the microneme contents. What cellular players are participating in this game of telephone that enables parasites to quickly transition modes? Lourido had helped to identify the next step in the process when he was a graduate student: the calcium signal activates a molecule called calcium-dependent protein kinase 1 (CDPK1). He found that CDPK1 is necessary for exocytosis, and so for the parasites to exit the host cell and travel. CDPK1 is not found in humans, which makes it a good possible drug target, as inhibiting it would only affect the parasite and not the host.
Now, using newer technologies, Chan developed an approach to identify the molecules that CDPK1 acts on—the next players in the game of telephone—helping researchers better understand the role of CDPK1 and gain a more complete picture of how T. gondii switches into spreading mode. CDPK1’s function is to attach a molecular structure called a phosphate group to other molecules. Chan identified 163 proteins that receive a phosphate group when CDPK1 is activated, some of which are likely to play roles in exocytosis. Chan also figured out which of the proteins CDPK1 targets directly, versus those that receive a phosphate group from another intermediary. Understanding what molecules CDPK1 targets, and what they do, will help researchers understand how a drug to inhibit CDPK1 would actually affect cells.
Narrowing in on a key player
One of the molecules that the researchers found to be acted on directly by CDPK1, a previously unidentified protein, caught Chan’s attention. Based on its similarity to molecules found in other species, Chan suspected that it was an activating adaptor: a molecule that helps connect cargo like micronemes to the cell’s conveyor belt and moves them along it. Chan named the molecule HOOK, after the similar molecules in other species. He also identified three key proteins that work with HOOK.
When Chan removed or deactivated HOOK, T. gondii parasites were still able to exit the host cell but were unable to travel or invade, compromising infection. This is consistent with the idea that HOOK is needed to move micronemes along the cell’s conveyor belt. Before the parasite receives the signal to switch modes, it already has a few micronemes locked and loaded at its tip, ready to release their contents outside of itself. Emptying these micronemes provides enough material to escape the host cell. However, without HOOK to help shuttle more micronemes to the cell tip, the parasite quickly runs out of material. T. gondii need to constantly release the sticky molecules found inside of micronemes as they move, almost like they are building a road as they travel along it. With no more micronemes, there’s no more road. The conveyor belt system that HOOK helps to activate is very good at rapidly and continuously shuttling materials forward to keep building new road.
The list of CDPK1 targets that the researchers compiled may contain other players that contribute to other aspects of the parasite’s transition between modes, but HOOK is clearly a major player, necessary for the parasite to succeed at traveling to new host cells. Chan and Lourido’s findings not only shed light on how parasites transition so quickly between modes, but could also provide new insights into how many species, including humans, move cargo inside of cells, as these species use similar cellular machinery to that found in T. gondii.
“Our work to understand how micronemes get shuttled across the cell really benefited from reading about work done on similar cargo trafficking in other species,” Chan says. “There are a lot of unanswered questions about how this sort of trafficking is regulated, and now I think T. gondii could be a good model to understand this more broadly.”
Alex W Chan, Malgorzata Broncel, Eden Yifrach, Nicole Haseley, Sundeep Chakladar, Elena Andree, Alice L Herneisen, Emily Shortt, Moritz Treeck, Sebastian Lourido (2023). "Analysis of CDPK1 targets identifies a trafficking adaptor complex that regulates microneme exocytosis in Toxoplasma." eLife 12:RP85654. https://doi.org/10.7554/eLife.85654.3
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu

