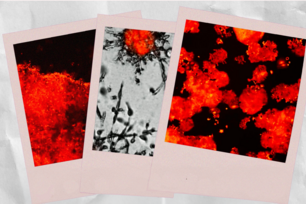
3D model reveals secrets of metastasis

Researchers tracked the movement of human prostate tumor cells, such as those below, in a 3D matrix. The gray and green images show software (Imaris) rendering of the cells, which changed shape as scientists altered their environment and increased the concentration of the anti β1 mAB 4B4 surface protein.
Images by Muhammad Zaman
CAMBRIDGE, Mass. (July 10, 2006) — A cancer cell breaks away from a primary tumor and settles in a new location, where it once again divides. Pharmaceutical companies typically use simplistic two-dimensional assays for this process, which is known as metastasis, to evaluate anti-cancer therapeutics. In these assays, cells crawl across the surface of a matrix, traveling in a single plane. But a new study indicates that this approach misses some crucial phenomena.
Working in the labs of Whitehead Member Paul Matsudaira and MIT professor Douglas Lauffenburger, postdoctoral researcher Muhammad Zaman discovered that cells move quite differently in three dimensions. His study, which focused on human prostate tumor cells, appeared this week in the online early edition of Proceedings of the National Academy of Sciences.
“Two-dimensional assays ignore the obstacles that cells face in their natural contexts,” explains Zaman, who recently became an assistant professor at the University of Texas at Austin. “In 3D, cells move through a thick jungle of fibers, or ‘vines’, that hinder forward progress.”
Cells must either squeeze through or chop up these putative vines to get anywhere. As a result, they move slower in three dimensions.
In an interesting twist, all cells need at least some vines to move, as they latch onto the “branches” with claw-like proteins called integrins and pull themselves forward. When Zaman disabled some of these claws, in a manner analogous to certain anti-cancer drugs, the cells moving across the top of the jungle canopy (in two dimensions) needed a greater number of vines to keep up their pace, while cells plowing through the jungle instead needed vines chopped to maintain the same speed. The complexity of this situation is further increased in that the cells become dramatically sensitive to the stiffness of the vines when the integrins are disabled and consequently tend to squeeze through the vines rather than pushing them aside.
“Our findings help explain why two-dimensional assays for metastasis-inhibiting drugs do not effectively predict their effects in tissue,” says Lauffenburger, who is director of MIT’s Biological Engineering Division. He believes pharmaceutical companies will eventually use three-dimensional assays, accompanied by appropriate computational models such as that also recently published by Zaman (in Biophysical Journal in 2005), to determine how drugs affect metastasis.
But technology must improve before more complicated 3D studies are attempted. For his 3D work Zaman worked with one sample at a time, using a special confocal microscope at the Whitehead-MIT BioImaging Center. The microscope divided each specimen into virtual slices, generating a new stack of images every 15 minutes.
“It took me about a year to get enough data because the microscope wasn’t designed for high-throughput experiments,” he says. Fortunately, the BioImaging Center has one of the most powerful sets of computers at MIT and the imaging processing and analysis went quite quickly.
“Muhammad was successful for two reasons,” says Matsudaira. “His computational model predicted what would happen in virtual experiments and then he was able to go straight to test the predictions with these complicated 3D experiments. As a result, the sophisticated models of cell movement enhance our understanding of key biological processes, including metastasis.”
The research was funded by the National Institutes of Health, the National Science Foundation and the Sokol Foundation for Cancer Research.
* * *
Paul Matsudaira’s primary affiliation is with Whitehead Institute of Biomedical Research. He also is a Professor of Biology and Bioengineering at Massachusetts Institute of Technology, and directs the Whitehead-MIT BioImaging Center and the Computational and Systems Biology Initiative (CSBi) at MIT.
* * *
Zaman, M. H., Trapani, L. M., Sieminski, A. L., Mackellar, D., Gong, H., Kamm, R. D., . . . Matsudaira, P. (2006). Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proceedings of the National Academy of Sciences, 103(29), 10889-10894. doi:10.1073/pnas.0604460103
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu