
Sharing the blueprints for the next generation
This article is part of our Building a Body story collection. Read the entire collection here.
Human body development starts with one fertilized egg cell, but that’s not really the beginning of the story. Long before sperm meets egg, the bodies that will make them have been preparing to create the next generation—in fact, they have been preparing since they themselves were embryos.
Early in embryonic development, our bodies, and those of other animals that reproduce sexually, set aside a group of cells known as germ cells to perform one task: in males, germ cells produce sperm and in females, they produce eggs. Unlike most of the body’s cells, germ cells have no essential role to perform to maintain the body; an animal can survive without its germ cells, it just cannot reproduce. Germ cells are also unique among cell types because they are the only ones whose descendants will survive after the body is gone. Our cells die with us, but the DNA from germ cells becomes the DNA in eggs and sperm, and thereby the DNA in any offspring. In this way, germ cells have a form of immortality. They are the only cells in sexually reproducing animals whose lines have continued, unbroken, since our biological history began. Their immortality also leads to rejuvenation: although each individual organism will age and die, when their germ cells create an offspring, the program of aging is reset, and the cells in the new body get to begin afresh.

Researchers at Whitehead Institute study germ cells to understand this immortality. How does the germline keep itself healthy? In order to produce fit eggs and sperm generation after generation, germline cells must continue to be high quality throughout their cell divisions. DNA must be maintained, because any mutations to the germline could either make the animal infertile, or could be passed on and affect the health and survival of offspring.
Whitehead Institute researchers also study how germ cells get set aside from the rest of the body’s cells early in development, and how the body carefully orchestrates their early activity so they develop properly. Germ cells must develop with precise timing, in specific moments and locations within a developing embryo, for fertility—and so the continuation of the species.
Creating the germ line
Early in embryonic development, the germline is set aside from the cells that make up the rest of the body, which are called somatic cells. The cells set aside are the predecessors of the mature germ cells that can make eggs or sperm, and these immature cells are called primordial germ cells.

Primordial germ cells (green) form early in development at the posterior tip of a fruit fly embryo.
Mariyah Saiduddin/ Whitehead Institute
Whitehead Institute Member and director Ruth Lehmann has uncovered many aspects of how germ cell fate is assigned by studying female fruit flies. As a graduate student, Lehmann identified and characterized genes essential to the proper formation of the germline. Germ cell precursors form at one tip—the back end—of an early fly embryo. Lehmann found that a gene she named Oskar plays a key role in assembling the necessary ingredients for germ cells in this location. Oskar helps to seed germ granules, droplet-like structures unique to the germline that form as RNA and proteins assemble together. Germ granules regulate the translation of RNA into protein, thereby controlling gene expression, and helping germ cells to form and develop properly.

Germ granules (red) in fruit fly primordial germ cells
Ruoyu Chen/ Whitehead Institute
Primordial germ cells form before the embryo begins making a reproductive tract. As the gonads—ovaries or testes—develop, the primordial germ cells must migrate through the embryo to reach them. Whitehead Institute researchers have helped discover how the cells are guided on this journey and mature into cells capable of making eggs or sperm. Lehmann’s lab has found factors involved in this migration. For example, they identified the signaling molecule that provides a trail of breadcrumbs for germ cell precursors to follow to their destination.
In recent work, Lehmann’s lab figured out how the fly’s body signals germ cell precursors to differentiate into mature germ cells in the right time and place—in the reproductive tract, once the ovaries are ready to host eggs—as well as what signals keep the precursors from maturing too early. Led by former Lehmann lab postdoc Torsten Banisch, the researchers identified swarm cells, whose function was previously unknown, as a critical relay that transmits cues to germ cell precursors that signal them to mature. If any of these processes goes awry—if the cells migrate to the wrong location, mature at the wrong time, or fail to receive the signal to mature—the fly is likely to lose its germline and become infertile.
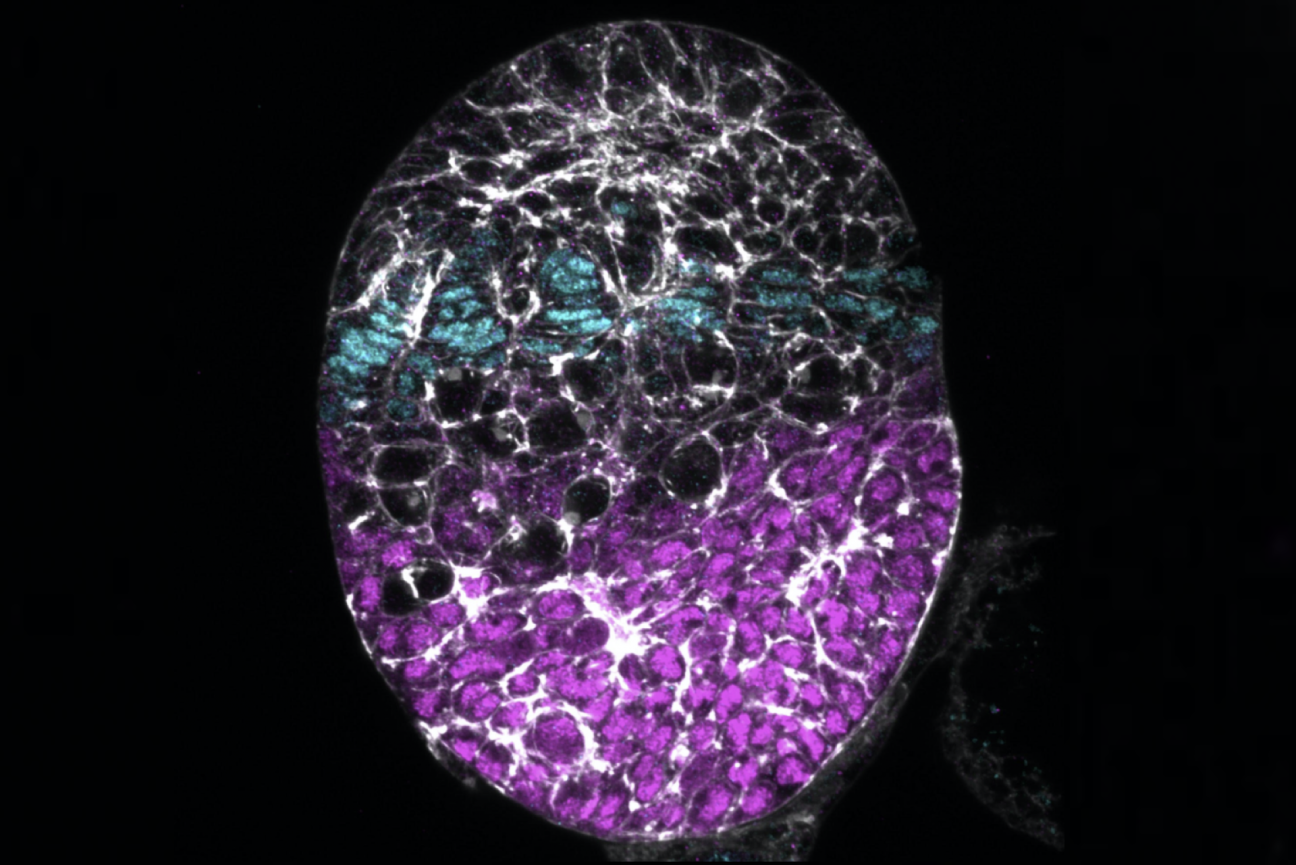
A developing Drosophila ovary with swarm cells are shown in magenta.
Torsten Bansich
Whitehead Institute Member David Page studies early germline development in mammals. When germ cell precursors are first set aside, they are still somewhat like stem cells, with flexible characteristics and fates. Researchers thought that the cells committed to their germline fate early on, but the Page lab found that the precursors only commit to becoming germ cells after they migrate to the gonads. Led by former postdoc Peter Nicholls, the Page lab found that the gene Dazl activates when the cells reach the gonads and prompts them to mature into germ cells. This discovery sheds light on the origins of testicular cancer, which can arise in young men. When a germ cell precursor remains in a stem-like state, it’s more likely to become cancerous. The researchers found that testicular cancer and—less commonly—some tumors in the female reproductive tract may come from germ cell precursors that fail to mature after reaching the gonads.

Primordial germ cells (red) are formed early in development and migrate to the gonad where they later undertake development to produce eggs or sperm.
Peter Nicholls/Whitehead Institute
This work from the Lehmann and Page labs shows how precisely the body must orchestrate germ cell migration and maturation in order to end up with healthy, mature germ cells. If germ cells do not receive and respond to the right mix of signals to guide them to the gonads and time their maturation with the developing body, it may lead to cancer or infertility. At a population level, proper germline development must be maintained, or else there will be no next generation to carry on the species. Figuring out how the germline stays healthy over many generations is also of interest to Whitehead Institute researchers.
Maintaining a healthy germline
We commonly think of cell division as one cell splitting into two identical copies. However, cells can divide asymmetrically, creating two daughter cells that are not identical to each other. Whitehead Institute Member Yukiko Yamashita studies germ cells in male fruit flies and has found that dividing germ cells pack one of the resulting cells full of only the best components at the expense of the other cell. For example, Yamashita found that one of the resulting cells will receive the other’s copies of certain genetic sequences that otherwise can be lost with age over the course of many cell divisions. The lab also found that chromosome pairs are non-randomly sorted into dividing germ cells, based on which has more copies of the genetic sequences that need to be maintained through generations. Their finding shows that this is one way the germline prevents loss of important genetic sequences with aging. The cell that gets all of the best materials continues dividing, keeping the germline healthy and fit for generations. Stem cells outside of the germline also divide asymmetrically, in order to allow some of their descendants to mature while others maintain the stem cell pool. Yamashita’s work with germ cells sheds light on this broader biology, delving into various mechanisms that enable asymmetric cell division.
For other cell types, sacrificing half of their cells in the way that the germline does to maintain its health would be too resource intensive to afford—every cell is needed to keep the body going—and so over many divisions our body’s cells degrade as we age. Germ cells are a small cell population that can afford to let only the cream of the crop survive—in fact, they need to be choosy in order for their descendants to keep dividing long after the rest of the body’s cells die. Discovering the processes like this that germ cells use to maintain their immortality could also provide insights into other immortal cell types, like cancers.
Lehmann has also uncovered some of the ways in which germ cells are able to maintain themselves throughout the generations. Her research illuminated how germ cells in flies protect their genomes from transposable elements or “jumping genes.” These are DNA sequences that can move to new positions in the genome, which can lead to mutations or change in genome size. Germ cells must be protected from such changes to their genomes, as these will either be passed down to offspring or could degrade the germ line to the point of infertility, putting an end to the cells’ immortality. Lehmann has discovered how germ cells prevent such changes.
Lehmann also studies inheritance of non-DNA components in the maternal germline. All germ cells must carefully maintain their DNA so it can be passed down to the next generation. The maternal germ line’s job does not end there, however; egg cells must pass on not only the mother’s DNA, but nutrients, starter organelles, and cellular components—such as RNA—that the earliest stages of the embryo rely on to function before they can ramp up production of their own components. One organelle of particular interest to Lehmann is the mitochondria, a structure that provides energy for cells. Mitochondria have their own genome, unique to them, and mutations to mitochondrial DNA can cause serious diseases. Lehmann’s lab studies how the maternal germline prevents passing on dangerous mutations in the mitochondrial genome.
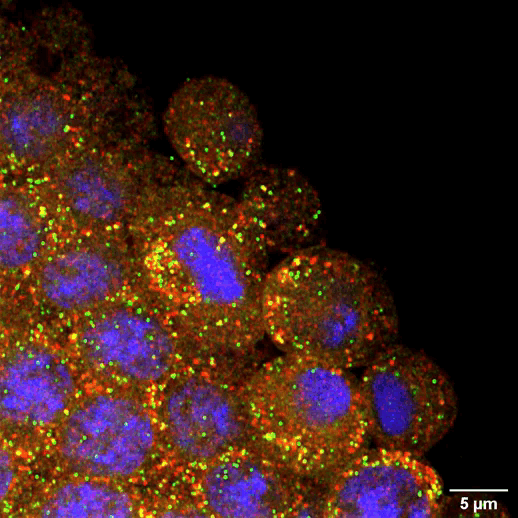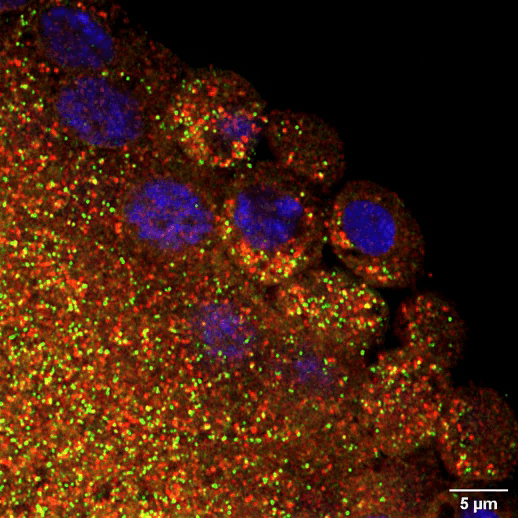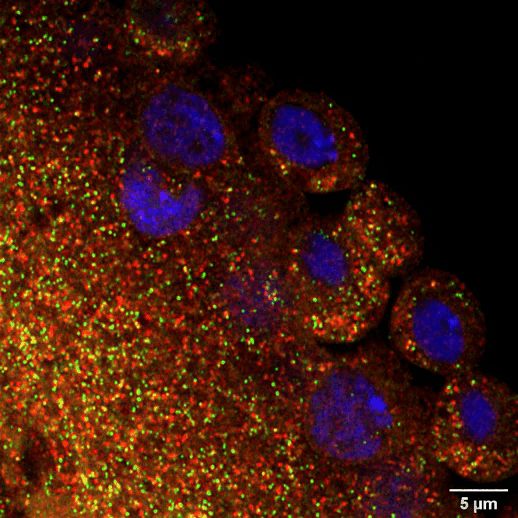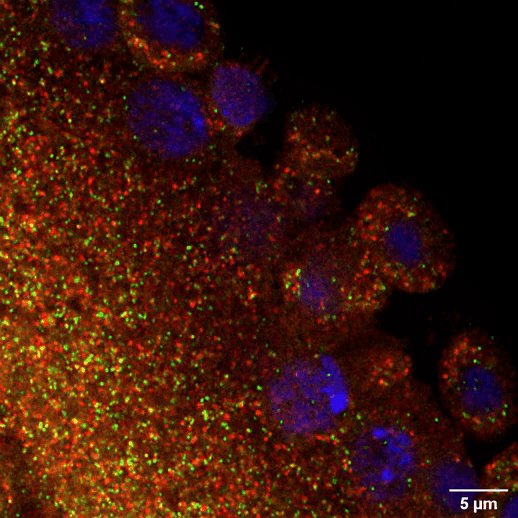
The maternal germline faces another challenge when it comes to long-term maintenance. In species like humans that take over a decade to reach puberty, immature egg cells must lie dormant for a very long time without degrading. By the time a woman is born, all of the egg cells she will ever have already exist in her ovaries. They sit there in arrested development for many years until one by one the body cues them to resume activity during ovulation and—perhaps—fuse with a sperm to begin building a new body. Whitehead Institute Member Iain Cheeseman and former postdoc Zachary Swartz were interested in how these cells retain the ability to divide after such a long period of dormancy. One critical part of the cell division machinery in cells is the molecule CENP-A: once cells lose it, they forever lose the ability to divide. Using starfish, Cheeseman and Swartz found that immature eggs constantly replace their CENP-A to keep their division machinery functional. Other cell types like muscle cells do not maintain their CENP-A in this way, and so they lose the ability to divide. This research demonstrates that the germline’s need for careful maintenance does not end with the creation of eggs and sperm. It also could provide insights into some forms of infertility.

Steven Lee/Whitehead Institute
From germ cell to embryo
Making sure that germ cells get set aside, develop correctly, and stay healthy over time is important for species’ survival. Embryonic development builds a whole body from a single fertilized egg and relies on germ cells to produce healthy eggs and sperm that are capable of carrying out this intricate progression. Work from Whitehead Institute researchers has delved into many important aspects of germ cell biology, helping us to understand these cells’ journeys and unique characteristics. Whitehead Institute researchers are excited to continue exploring the biology of germ cells in order to learn more about how our bodies prepare for the monumental task of building a body.
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


