Structure of RNAi complex now crystal clear
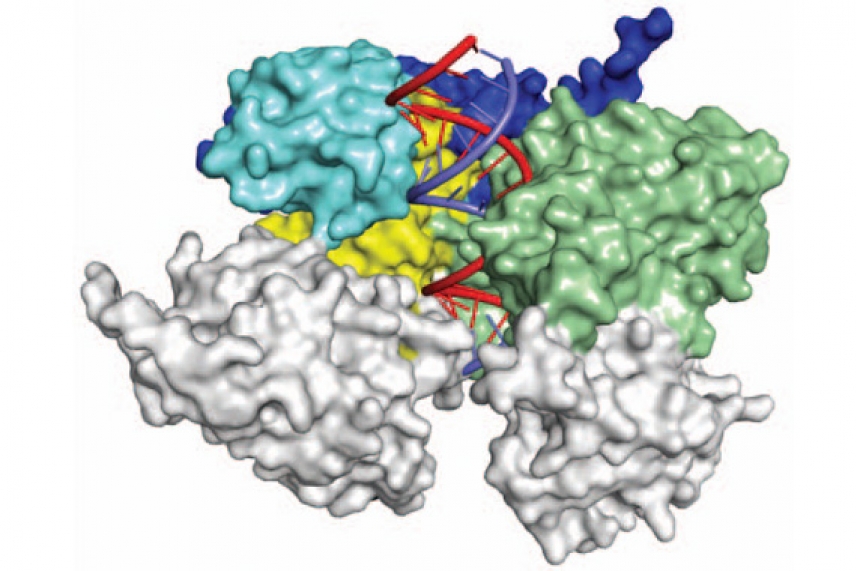
Model of eukaryotic argonaute protein
Courtesy of Nature 486, 368–376
CAMBRIDGE, Mass. – Researchers at Whitehead Institute and Memorial Sloan-Kettering Cancer Center have defined and analyzed the crystal structure of a yeast Argonaute protein bound to RNA. This complex plays a key role in the RNA interference (RNAi) pathway that silences gene expression. Describing the molecular structure of a eukaryotic Argonaute protein has been a goal of the RNAi field for close to a decade.
“You can learn a lot from biochemical experiments, but to more fully understand a protein like Argonaute, it’s useful to know where all of the atoms are and which amino acids are playing important roles,” says Whitehead Institute Member David Bartel, who is also an MIT professor of biology and a Howard Hughes Medical Institute (HHMI) investigator. “Learning the Argonaute crystal structure is an important step in understanding the RNAi biochemical pathway and will be the basis for many future experiments.”
The yeast Argonaute structure is described in the June 21st print issue of Nature.
In humans and most other eukaryotes, the RNAi pathway can reduce cellular protein production by reducing the proteins’ RNA templates. By exploiting this pathway, scientists are able to knock down the expression of specific proteins and thereby determine their roles within the cell or organism. The RNAi pathway has also been of considerable interest for the treatment of human disease.
RNAi depends on two proteins, Dicer and Argonaute. Dicer recognizes double-stranded RNA (dsRNA), latches onto it, and chops it into pieces 21-23 nucleotides long. Argonaute recognizes the dsRNA bits, discards one strand, and uses the other as a guide. When a single-stranded RNA matches the guide RNA’s sequence, Argonaute cleaves the targeted RNA, thereby preventing it from serving as a template for protein production.
To determine the structure of Argonaute, Bartel and graduate student David Weinberg partnered with Kotaro Nakanishi in Dinshaw Patel’s lab at Sloan-Kettering. Although the team expected to solve the structure of Argonaute alone, they were surprised to find that the protein came along with small bits of RNA that were also observed in the structure. The incorporation of these RNAs had switched the protein into an activated state that contained a four-component active site, the identification of which solved a longstanding mystery of what constituted the “missing” fourth component. With the structure of this complex in hand, scientists now have a better understanding for how it works.
“Seeing the crystal structure of a eukaryotic Argonaute for the first time was very exciting—it’s such a large protein with a complicated topology and many moving parts,” says Weinberg. “It’s a really impressive molecular machine.”
This work was supported by National Institutes of Health (NIH), the Human Frontier Science Program, the Japan Society for the Promotion of Science, and the National Science Foundation (NSF).
* * *
David Bartel is a Member at Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a Howard Hughes Medical Institute Investigator and a professor of biology at Massachusetts Institute of Technology.
* * *
Citation:
Nakanishi, K., Weinberg, D. E., Bartel, D. P., & Patel, D. J. (2012). Structure of yeast Argonaute with guide RNA. Nature, 486(7403), 368-374.
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


