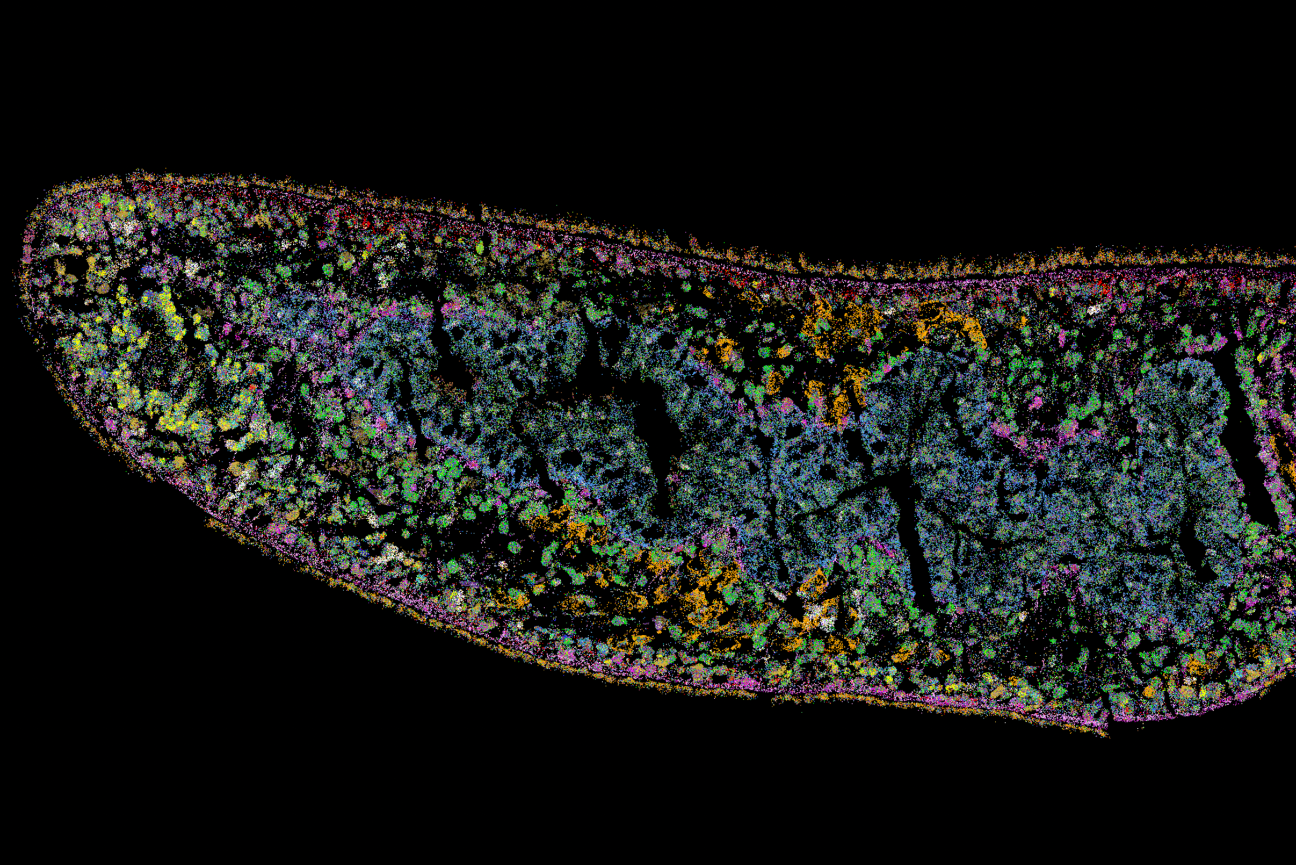
A MERFISH image showing the anterior region of a planarian section. Each spot represents the detection of a single mRNA molecule, and each color represents the expression of a different gene.
Courtesy of Chan Park
Cell fate choice during adult regeneration is highly disorganized, new study finds
A team of scientists at the Whitehead Institute for Biomedical Research and MIT has spatially mapped the choices stem cells make during tissue regeneration in flatworms, revealing an unexpected finding: Rather than being organized into homogeneous neighborhoods — where one group of stem cells becomes eye cells and another becomes muscle cells, for example — the spatial pattern of stem cell choice is highly heterogeneous, with adjacent stem cells choosing different fates. This new insight, described in the November 16 online issue of Nature Communications, emerges from the systematic application of a powerful single-cell method, known as MERFISH, which allowed the team to simultaneously visualize the choices of hundreds of individual stem cells.
“What we found is really surprising,” said senior author Peter Reddien, Member of Whitehead Institute, professor of Biology at Massachusetts Institute of Technology, and investigator with the Howard Hughes Medical Institute. “There appears to be very little spatial organization in how stem cells choose their fate. Cells can choose to become an eye or skin or gut cell all right next to each other in a completely jumbled, messy pattern. This has important implications for how we understand the mechanisms that underlie these cell fate decisions.”
The regenerative planarian flatworm, Schmidtea mediterranea, is one of a group of animals that possess a kind of biological superpower — adult organisms that can fully regenerate missing or damaged body parts. This capability is made possible by specialized stem cells, called neoblasts, which can give rise to a wide array of mature cell types. In adult planaria, there are over 125 different types of cells. But how do stem cells select their biological fates with such a broad menu of options? And how are these choices organized spatially within the animal?
To answer these questions, the team, including co-first authors Chan Park and Kwadwo Owusu-Boaitey, turned to a collection of methods broadly known as spatial transcriptomics. These methods allow scientists to visualize cells at high resolution using the molecular signatures of gene activity (called “transcripts”) to label different types of cells.
“How do cells decide what to become? This is a longstanding, fundamental question in biology,” explained Reddien. “Lots of beautiful work over many decades has been done to understand this question in a developmental context. But adult regeneration is a very different case, and spatial transcriptomics presented a powerful way to characterize it.”
After piloting different spatial transcriptomic methods, the team chose MERFISH (multiplexed error-robust fluorescence in situ hybridization), which can simultaneously detect hundreds of different transcripts at single-cell resolution in tissue sections. That capability allowed the researchers to map the spatial organization of stem cells as well as the full suite of adult cell types they can become.
“Spatial transcriptomics is a rapidly emerging field of technology,” said co-first author Chan Park. “It is a transformative set of methods, and this work represents one of the first uses of MERFISH to understand cell fate choice in adult tissue regeneration.”
To conduct their MERFISH experiments, the researchers used a wide array of molecular probes, which detect transcripts present in different cell types. These include stem cells that have begun to commit to their cell fate choice and cells that comprise adult tissues, such as muscle, skin, intestine, nerve, and pigment cells. With this diverse assortment of molecular probes, the team created a map of cell fate choice in the regenerating adult flatworm, which revealed a surprising lack of organization.
“It was surprising to see just how little the actual local neoblast neighborhood impacted the cell's fate choice,” said co-author Giselle Valdes. “This highlights the significance of roles other than local position in this process.”
“What excites me most about this study is that it's uncovered core properties behind how the stem cells in a regenerating organism contribute to regeneration, while also leading to new questions,” said co-first author Kwadwo Owusu-Boaitey.
The researchers’ discovery has several important implications. First, the lack of pattern suggests that stem cell fate choice is not predominantly directed by local external signals. Instead, it seems the choices are largely left to the stem cells themselves. “We hypothesize that there could be an element of randomness, like a weighted dice roll, which I think is a very exciting possibility to explore,” said Reddien.
In addition, regenerated tissues are always highly organized. So, order must somehow emerge. Reddien and his colleagues believe that pattern arises as a result of cell migration — after the stem cells have made their choices, their descendants likely sort themselves in an orderly fashion. Future work is needed to test these ideas and also to understand the molecular underpinnings that underlie the disorganization of cell fate choice in flatworm regeneration.
Citation:
Park, C., Owusu-Boaitey, K.E., Valdes, G.M. et al. Fate specification is spatially intermingled across planarian stem cells. Nat Commun 14, 7422 (2023). https://doi.org/10.1038/s41467-023-43267-2
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu