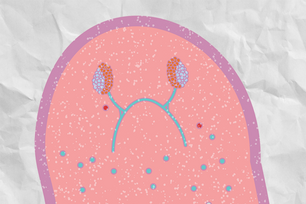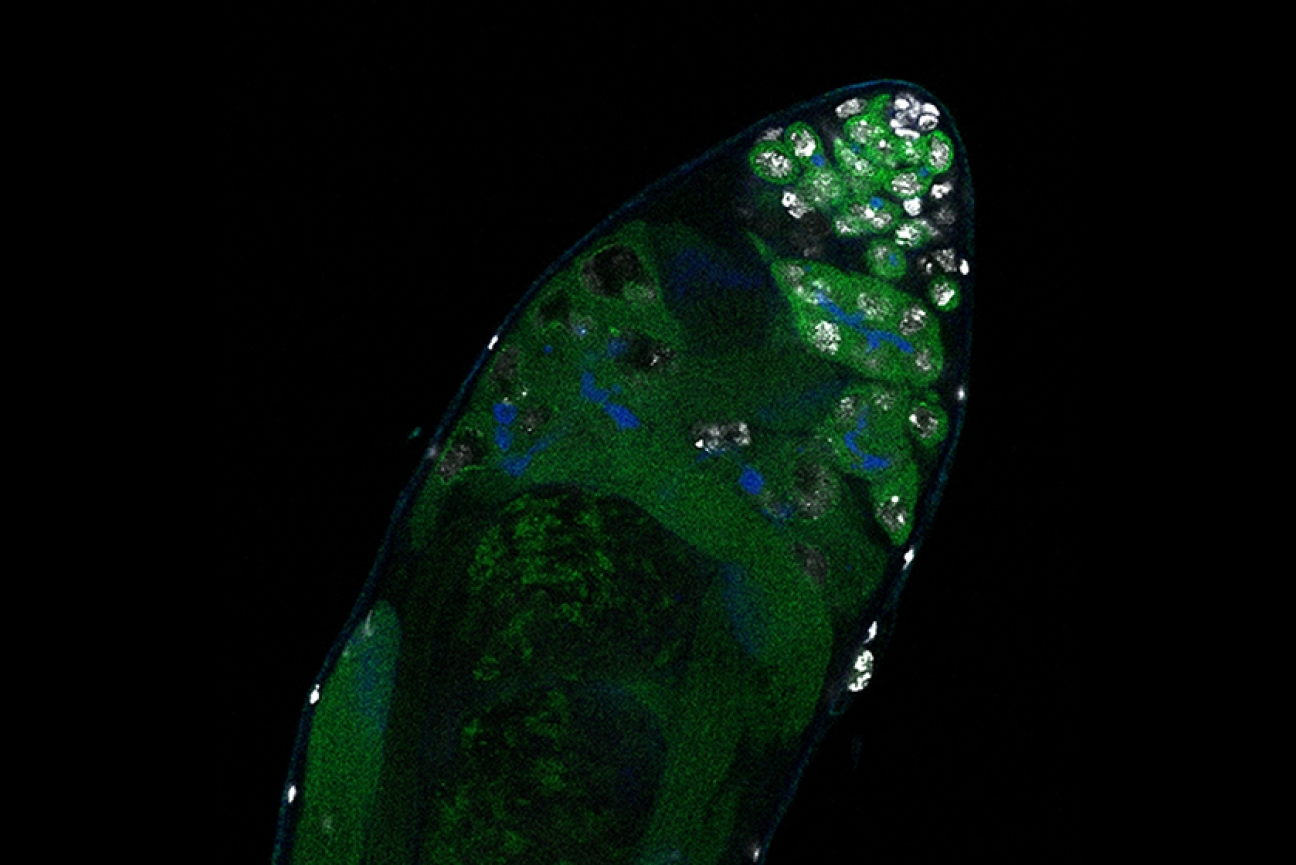
Fruit fly testis with a reduced number of differentiated germ cells (seen in the upper right hand of the testis).
Adapted from Figure 5, Jonathan O. Nelson, Tomohiro Kumon, Yukiko M. Yamashita. "rDNA magnification is a unique feature of germline stem cells." PNAS, November 15 2023. https://doi.org/10.1073/pnas.2314440120
Maintaining fertility requires uneven division of DNA
Germline stem cells are the pool of stem cells capable of becoming eggs or sperm. They divide asymmetrically, such that one of the cells resulting from a division is another stem cell and the other is a differentiated cell, which has progressed one step further down the path towards becoming an egg or sperm. Researchers have thought that this asymmetrical division served to replenish the pool of stem cells—making sperm or eggs, but also making more stem cells to produce future sperm or eggs. However, the germline has another way to replenish itself: cells that have differentiated only one or two steps down the path to becoming eggs or sperm are capable of reverting into stem cells. Why, then, do stem cells divide asymmetrically?
New research from Whitehead Institute Member Yukiko Yamashita, who is also a professor of biology at the Massachusetts Institute of Technology and an HHMI Investigator, and former postdoc in her lab Jonathan Nelson shows that asymmetrical division in germline stem cells serves a different but equally important purpose in male fruit flies (Drosophila melanogaster), a common model animal for germline research. The work, published in the journal Proceedings of the National Academy of Sciences (PNAS) on November 13, suggests that in flies, germline stem cells divide asymmetrically in order to unequally split a certain kind of DNA, called ribosomal DNA (rDNA), between the two dividing cells and then keep the cell with more rDNA in the stem cell pool. This is necessary in order to keep the germline viable over generations of cell divisions, and so to keep individual flies fertile and capable of reproduction. The researchers show that only germline stem cells, and not other types of germ cells, drive this process, and explain why stem cells’ asymmetric divisions make them uniquely suited to maintaining rDNA.
Cells must maintain rDNA to be viable and fertile
Ribosomal DNA is critical to maintain in the germline because it contains the instructions for making a major part of ribosomes, the cellular machines that build proteins from genetic instructions. Proteins are the main workhorses of the cell, and so cells need to make many ribosomes in order to build all of the proteins that they need. Consequently, rDNA exists as many copies repeated in a row of the code for components of the ribosome. All of these repeats make it easy for the cell to mass produce ribosomes, but they also come with a risk: repetitive DNA is prone to losing repeats during cell division. When the cell’s rDNA is copied, it’s easy for a few of the many identical repeats to get cut out, so that the resulting copy of the genome has fewer rDNA repeats than the original.
Most cells can afford to lose a few rDNA repeats without too many negative effects, but the germline cannot. Whereas other cells die with the body they are in, germ cells produce eggs and sperm that will form a new body, which produces new germ cells, and so on. The germ cell lineage is effectively immortal. Over the course of its endless cycle of cell division, the loss of rDNA repeats would add up until the cells became dysfunctional and then died. This would make the individual bearing those germ cells infertile, and so cause their lineage to go extinct.
A role for a “selfish” parasite in maintaining rDNA
Researchers have known that germ cells have some way to regain rDNA repeats when the number gets too low—if germ cells couldn’t do this, none of us would exist—but the details of how cells achieve this have been largely mysterious. One proposed model was that when a germ cell divides, sometimes it might divide up its rDNA unequally between the two resulting cells, so that one cell would gain rDNA repeats. Yamashita and Nelson have previously found evidence that this model is correct, and they discovered some of the specific mechanisms that enable it to happen. In a 2023 PNAS paper, the researchers showed that a retrotransposon, a “selfish” genetic element whose function is to make more copies of itself, actually helps germ cells maintain rDNA. During cell division, the retrotransposon R2 slices open one copy of the chromosome containing rDNA in its quest to insert extra copies of itself into the genome. The cell tries to repair the break using the copy on the other intact chromosome, but the tricky nature of repetitive DNA can cause the cell to lose its place, so that it stitches a stretch of rDNA repeats from one copy of the chromosome into the other copy instead.
Through this process, the germline can boost the level of rDNA in a cell—but only by as much as another cell loses. How does this win-lose exchange lead to an overall increase in rDNA levels across the germline cell population to compensate for lost rDNA? In this latest work, Yamashita and Nelson show through mathematical modeling that in cells that divide symmetrically, it would not. Gains and losses in rDNA through this form of exchange would occur essentially at random and cancel each other out over time.
In praise of asymmetry
Now consider an asymmetric division. After a germline stem cell divides, the cell that differentiates will go through a few more divisions and ultimately create a specific number of sperm cells–the number happens to be sixty-four. If this daughter cell gets the chromosome with more rDNA repeats, then that would lead to sixty-four sperm with more rDNA repeats—but that would be it, as the sperm have exited the pool of replicating germline stem cells.
However, the daughter cell that remains a germline stem cell will divide again to create a differentiated cell (which will become sixty-four sperm) and another stem cell, which will divide again, leading to another sixty-four sperm and another stem cell—and so on. All of these cells, including many sperm, would inherit the higher number of rDNA repeats. Furthermore, at each division, there would be an opportunity for another unequal split of rDNA. As long as the stem cell always gets the boost in rDNA, then the cumulative number of rDNA repeats would keep growing in the overall population over time—and Yamashita and colleagues’ past work shows that the germline can ensure this. A 2022 Science Advances paper from Yamashita and then-postdoc in her lab George Watase showed that when a germline stem cell divides, the DNA strand with more rDNA repeats is tagged with a protein that the researchers named Indra, which helps mark it to stay in the daughter cell that will become another stem cell. Yamashita and Nelson’s new paper includes mathematical modeling by second author Tomohiro Kumon, a postdoc in Yamashita’s lab, that proves that this is not only sufficient to restore the level of rDNA repeats over time, but that it is the most effective and efficient way for the germline to do so.
“There was this problem with the unequal exchange model of rescuing rDNA, because every cell that gained rDNA did so at the expense of another that was losing it,” Nelson says. “What we show here is that the reason why there's a bias towards gain in the germline is because this process is happening within these asymmetrically dividing germline stem cells that can gain and gain and gain, while the cells that lose rDNA exit the cycle and so have a limited effect.”
The researchers complemented their mathematical modeling with evidence that the process to increase rDNA repeats occurs primarily or solely in germline stem cells. They found that when the number of rDNA repeats got low enough, then expression of R2 and the presence of double-stranded DNA breaks both increased in germline stem cells, but not significantly in other germ cell types.
Yamashita and Nelson propose that the different cell types in the germline take on different functions to create a pipeline for maximizing the health of future sperm. Germ cells that are one or two steps down the path of differentiation from stem cells are essentially identical to them, to the point that they can be difficult to tell apart in testing, but they divide symmetrically. They are also much more sensitive to DNA damage; the researchers found that R2 exposure kills these cells.
Germline stem cells, with their asymmetrical division and ability to tolerate R2 expression, serve to restore rDNA levels when they get too low. Then the differentiated germ cells serve to weed out mutations—including those introduced during R2 expression in the earlier stem cell stage—by killing off cells with DNA damage. The different strengths of the different types of germ cells creates an effective pipeline to produce the largest number of sperm cells with high rDNA repeat number and low DNA damage.
Eventually, this new understanding of the details of how cells maintain their rDNA could lead to medical therapies. For example, cancer cells are, like germ cells, an essentially immortal cell line, and so must have a way to maintain their rDNA. If researchers could someday find a way to prevent them from doing so, that could be a good treatment strategy. The work also may have implications for research on aging, as rDNA decreases with age in other cell types. In the meantime, Yamashita and Nelson are excited to have solved several long-standing mysteries in their field, including how germ cells can restore rDNA at a population level when each division creates an equal loss and gain of rDNA, and why germline stem cells divide asymmetrically.
“Typically, when you publish a paper, you feel like you’ve fit two puzzle pieces together, but in this case, I feel like we fit a bunch of puzzle pieces together,” Yamashita says. “It’s been immensely satisfying to find answers to multiple questions and see how they all fit together to explain the mechanisms of this process that’s necessary for germline immortality.”
Citations:
Jonathan O. Nelson, Tomohiro Kumon, Yukiko M. Yamashita. "rDNA magnification is a unique feature of germline stem cells." PNAS, November 15 2023. https://doi.org/10.1073/pnas.2314440120
Jonathan O. Nelson, Alyssa Slicko, Yukiko M. Yamashita. "The retrotransposon R2 maintains Drosophila ribosomal DNA repeats." PNAS, May 30, 2023. https://doi.org/10.1073/pnas.2221613120
George J. Watase, Jonathan O. Nelson, Yukiko M. Yamashita. “Nonrandom sister chromatid segregation mediates rDNA copy number maintenance in Drosophila.” Science Advances, July 27, 2022. https://www.science.org/doi/10.1126/sciadv.abo4443
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu