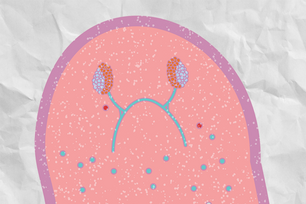
Whitehead Member Peter Reddien has created a transcriptome atlas for the planarian flatworm.
Christopher Fincher/Whitehead Institute
How one worm can rebuild its whole body
This article is part of our series on rejuvenation research at Whitehead Institute. Click here to view the entire collection.
As we age and incur injuries, we often accumulate permanent scars. If we lose a body part to injury, that part is gone forever. However, some animals have no trouble recovering from even the most grievous injuries. Planarians, a type of aquatic flatworm, are one such super regenerator, able to restore their bodies by growing back anything from an eye or a tail to a head. If you cut a planarian in half, two new planarians will grow out of the pieces.
Whitehead Institute Member Peter Reddien seeks to understand how planarians (Schmidtea mediterranea) are able to regenerate so effectively. Researchers in his lab have uncovered many of the mechanisms and principles of regeneration. This work may prove useful in the field of regenerative medicine, which aims to promote better healing or even regrowth in people.
“The more we understand about the mechanisms underlying regeneration in animals that naturally can accomplish it, the better we will understand the basis for the limitations of our own regenerative capacity and whether we might improve upon it,” Reddien says.
In pursuit of such understanding, Reddien and his lab members have discovered how planarians initiate regeneration, how they ensure that lost parts grow back in the right places, how their stem cells make choices about what cell type to become, and more.
Initiating regrowth
After an injury, animals have a wound healing response that triggers the body to seal off the open wound and repair the immediate area. In animals that cannot regenerate, the response ends there, but in planarians, healing transitions into regeneration, restoring any lost parts. The Reddien lab identified a gene, active in the skin, that grows to cover the wound site, that plays a key role in triggering this transition in planarians. If the gene, which the researchers called equinox, is not active after large injuries, the wound site will still heal but the lost body parts will not regenerate. This finding expands researchers’ understanding of the cascade of events that has to happen for regeneration to take place.

The equinox gene is expressed around the perimeter of an uninjured planarian flatworm (purple). After an injury, equinox plays a key role in initiating regeneration.
M. Lucila Scimone
Maintaining a body map
How do planarians know where to regrow missing body parts? In order to regenerate missing body parts in the right places, the planarian’s cells follow a body plan blueprint that is maintained by what are called position control genes (PCGs). These genes function as a sort of GPS system, directing the cells in where to go and what to become. Reddien’s lab discovered that in planarians, PCGs are active in muscle tissue. They later found that PCGs are also active in muscle tissue in three banded panther worms (Hofstenia miamia). The fact that muscle plays the same role in both species suggests that it also played the same role in their last common ancestor, which existed more than 550 million years ago. That common ancestor was also an ancestor to humans and many other animals, which indicates that muscle tissue was a source of positional information in our ancestry too.
Since discovering the role of muscle in maintaining the body’s GPS grid, researchers in Reddien’s lab have discovered more details of how this system works. One question that Reddien had is how cells adjust during the period right after an injury, when there is disagreement between the body’s blueprint and its actual anatomy. If a worm gets cut in half, the GPS grid recalibrates quickly and anatomy adjusts to this new grid slowly. For example, a head fragment must start generating “tail” signals at the wound, and the “head” patterning information shifts away from the wound. While the “head” signal is in flux, how does the planarian know where to send stem cell progeny to maintain its eyes?
Researchers in Reddien’s lab found that the cells involved in regrowing the body follow a set of rules to solve such dilemmas. For example, in the case of eyes in a head, eye progenitor cells that come close to an existing or already-growing eye will join that eye, rather than continuing farther forward. This prevents the planarian from repeatedly starting to regrow eyes in new locations as the GPS grid adjusts. The cells rely on a combination of anatomical self-organization and positional signals to control where they rebuild and maintain tissues. The researchers were able to manipulate these factors to create planarians with misaligned or extra eyes.
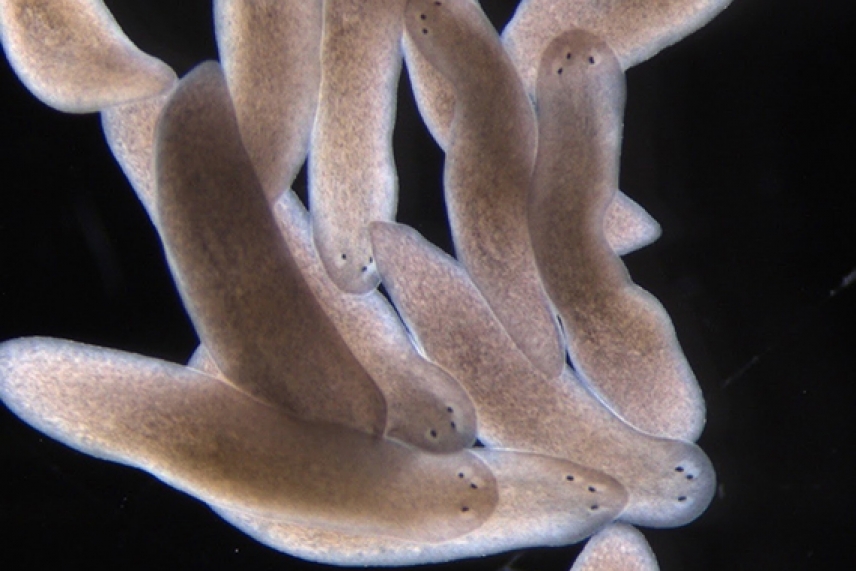
Three-eye planarians generated by a simple surgical trick revealing self-organizing dynamics that occur during regeneration.
Kutay Deniz Atabay/Whitehead Institute
Building on what they learned about the principles that govern eye regrowth, the researchers discovered how the planarian rewires the connections between new eyes and the brain. Axons are the wires between the eye and brain, and they must route their way through the regrowing body to connect the two. Most animals have so-called “guidepost cells” that exist during their embryonic development to help guide axons in the right direction, but these cells are typically not present in adulthood, when they are no longer needed. Researchers in the Reddien lab found that planarians maintain guidepost cells as adults, so that axons can always find the right route. The researchers also found that guidepost cells are muscle cells in planarians, rather than neural cells as is more typical in development, once again revealing a key role for muscle as the source of adult positional information in planarians.
Staying primed for regeneration
Planarians have a pool of stem cells, called neoblasts, that can divide to become any cell type. Researchers anticipated that these cells might follow a typical funnel-like progression in their divisions, going through several intermediate stages on the way to their final cell type, with each division narrowing the options of what they can become. For example, in humans, a stem cell may become a blood cell progenitor, and then an immature white blood cell, on its way to becoming a white blood cell. However, Reddien and colleagues found that stem cells in planarians remain flexible in their fates. The cells make large, single jumps between a naive state and final fate choice–to make skin, gut, neuron, or something else–in the course of a single division. Neoblasts can divide asymmetrically, which allows them to switch paths rather than becoming stuck in a narrowing funnel of options. This flexibility helps planarians to regenerate by making it easy for the animal to generate any cell type rapidly from a pool of starting stem cells.
Building the resources to learn more
Reddien and his colleagues continue to study aspects of planarians’ incredible regenerative abilities that are not fully understood. To help in that work, they have built a map, or cell atlas, of gene expression for every cell type in the planarian body. This required sequencing tens of thousands of cells, something that has only recently become feasible thanks to advances in technology. This complete planarian cell type gene expression atlas was the first of its kind for any adult organism. The researchers intend to use this atlas to dive even deeper into the principles governing regeneration.
“We’re still in the early phases of realizing the potential of these tools,” Reddien says. “Now we can apply them to many different problems in regeneration.”
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu