Brain development takes an unexpected bend
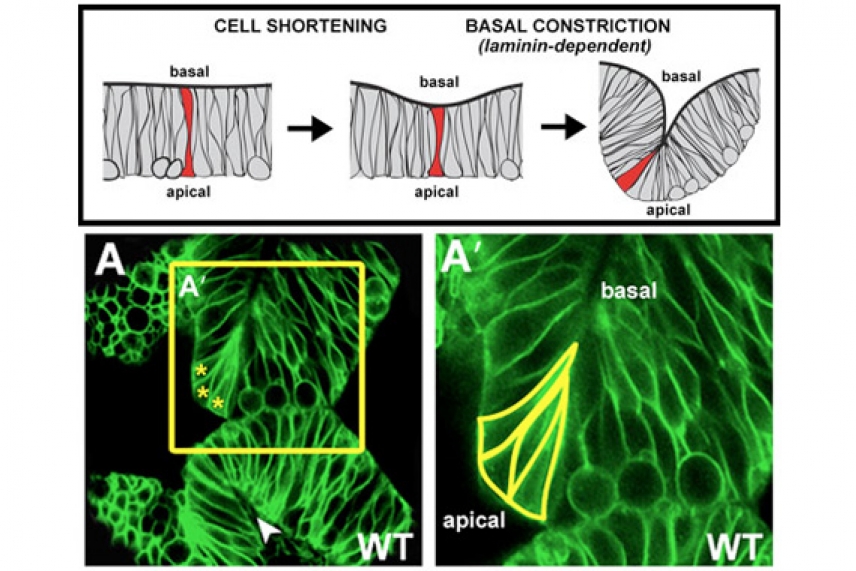
As a zebrafish’s brain begins to bend, the process is driven from the outside (basal) side, and the cells on the apical surface (outlined in yellow in the image on the right) become wedge-shaped. This basal-constriction process is likely to be widely conserved among organisms, and helps to explain more broadly how sheet-like tissues are folded in developing organisms.
Image: Ellie Graeden/Whitehead
CAMBRIDGE, Mass. — Organs, such as the brain or the heart, are not simply collections of the correct types of cells that are required for their function. If these cells are not organized and shaped properly, the organ does not function. One crucial part of this organization is to bend sheets of connected cells ("epithelia") so that they form a structure that is an integral part of the functional organ.
Researchers in the lab of Whitehead Member Hazel Sive have moved one step closer to understanding how this process works in properly shaping the embryonic brain. Their findings, published in the November/December issue of Mechanisms of Development, reveal a process called "basal constriction" during which cells of the neural tube’s epithelium constrict on the outside to actively shape a conserved and crucial early fold in the brain.
An epithelium has two sides, explains Sive. One side of the cell sheet is called "apical", the other "basal", and each contains distinct proteins.
“Many studies have described bending of the apical side of the cells in a sheet,” she notes. “The apical surfaces get smaller relative to the basal surfaces and this causes the sheet to bend. This is very important in forming tubes, which are found in essentially all organs, and constrictions, which bend many organs in characteristic ways. Apical constriction is widely studied and the molecular underpinnings quite well understood."
Surprisingly, however, no one has described the inverse process, in which the basal side of the cells constricts to bend the cell sheet.
"We are interested in the genes underlying early brain structure, and the connections to devastating birth defects such as anencephaly and hydrocephalus,” says Sive.
“One of the first ‘great bends’ of the brain is called the midbrain-hindbrain boundary constriction and this is essential for normal brain structure,” says Sive. "We noticed that this bend formed in an unusual way, with the basal sides of the cell sheet constricted."
Researchers in the Sive lab use the zebrafish as a tool to understand human brain development, as early brain development in the fish and human are virtually indistinguishable. However, the zebrafish is transparent, enabling investigators to look into the living brain, and watch how cells move and change shape in real time.
Sive lab investigators looked at the midbrain-hindbrain boundary constriction in the living zebrafish brain, after labeling the membrane of each cell in the constriction with a green fluorescent protein. Then, using time-lapse microscopy techniques, postdoctoral scientist Jennifer Gutzman observed the shape changes of individual cells as the neural tube constricted and dilated to form the early embryonic brain.
"We followed single cells over time to see what happens during brain development, and found that a distinct group of cells at the midbrain- hindbrain boundary constrict basally to form the sharp bend," says Gutzman, co-lead author on the paper.
This was an intriguing result, suggesting that the "inside-out" basal constriction was involved in making the bend. "However, we wondered if this constriction formed passively, as brain cavities (called "ventricles") inflated with fluid and pushed the tissue so that it basally constricted," notes Ellie Graeden, co-lead author and a graduate student in the Sive lab.
But the researchers found that basal constriction takes place even in zebrafish mutants that fail to inflate their cavities, so that folding of the tissue is actively controlled, at least in part, from the basal surface.
"There are four cells that are pivotal at the midbrain-hindbrain boundary for basal constriction," says Sive. "These few cells cause a very dramatic shape change to form a deep constriction in the epithelium."
In addition, Sive lab researchers demonstrated that this process requires the protein laminin, which is a crucial part of the basement membrane that lines the outside of the neural tube.
Sive adds that preliminary evidence suggests that the newly described basal constriction phenomenon is likely to be widely conserved, and helps to explain more broadly how sheet-like tissues are folded in developing organisms. "Our challenge now," says Sive, "is to figure out how cells know to basally constrict, and what genes are required for the process. Whatever results we obtain will be novel, and that is a very exciting place to be."
* * *
Hazel Sive’s primary affiliation is with Whitehead Institute for Biomedical Research, where her laboratory is located and all her research is conducted. Sive is also a professor of biology at Massachusetts Institute of Technology.
* * *
Gutzman, J. H., Graeden, E. G., Lowery, L. A., Holley, H. S., & Sive, H. (2008). Formation of the zebrafish midbrain–hindbrain boundary constriction requires laminin-dependent basal constriction. Mechanisms of Development, 125(11-12), 974-983. doi:10.1016/j.mod.2008.07.004
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu



