Reprogrammed fibroblasts identical to embryonic stem cells

Mouse containing cells derived from a reprogrammed fibroblast
Image: Sam Ogden
CAMBRIDGE, Mass. — Embryonic stem cells are unique because they can develop into virtually any kind of tissue type, an attribute called pluripotency. Somatic cell nuclear transfer ("therapeutic cloning") offers the hope of one day creating customized embryonic stem cells with a patient's own DNA. Here, an individual's DNA would be placed into an egg, resulting in a blastocyst that houses a supply of stem cells. But to access these cells, researchers must destroy a viable embryo.
Now, scientists at Whitehead Institute have demonstrated that embryonic stem cells can be created without eggs. By genetically manipulating mature skin cells taken from a mouse, the scientists have transformed these cells back into a pluripotent state, one that appears identical to an embryonic stem cell in every way. No eggs were used, and no embryos destroyed.
“These reprogrammed cells, by all criteria that we can apply, are indistinguishable from embryonic stem cells,” says Whitehead Member and MIT professor of biology Rudolf Jaenisch, senior author of the paper that appeared online June 6 in Nature.
What's more, these reprogrammed skin cells can give rise to live mice, contributing to every kind of tissue type, and can even be transmitted via germ cells (sperm or eggs) to succeeding generations. "Germline transmission is the final and definitive proof that these cells can do anything a traditionally derived embryonic stem cell can do," adds Jaenisch.
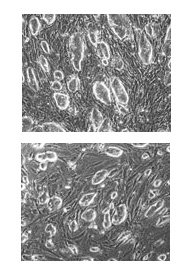 |
|
|
Naturally derived embryonic stem cells from mice (top) are morphologically identical to reprogrammed fibroblasts (bottom). Images: Marius Wernig |
Two additional papers report similar findings. The first, by Shinya Yamanaka of Kyoto University, will be published in the same issue of Nature. The second, from Konrad Hochedlinger, formerly of the Jaenisch lab and now at the Center for Regenerative Medicine at Massachusetts General Hospital and Harvard Stem Cell Institute, will appear in the inaugural issue of the journal Cell Stem Cells. Additionally, another paper in Nature from Kevin Eggan, also of the Harvard Stem Cell Institute and a former member of the Jaenisch lab, describes using mouse zygotes, rather than eggs, for somatic cell nuclear transfer.
Jaenisch cautions that "all these results are preliminary and proof of principle. It will be awhile before we know what can and can't be done in humans. Human embryonic stem cells remain the gold standard for pluripotent cells, and it is a necessity to continue studying embryonic stem cells through traditional means."
In August 2006, a team of researchers at Kyoto University led by Yamanaka reported a landmark discovery that by activating four genes in a mouse skin cell, they could reprogram that cell into a pluripotent state resembling an embryonic stem cell. However, the resulting cells were limited when compared with real embryonic stem cells, and the Kyoto team was unable to generate live mice from these cells.
A team of researchers decided to replicate this experiment, while refining certain technical aspects. This group was led by Jaenisch lab postdoctoral researchers Marius Wernig, Alexander Meissner and Tobias Brambrink; graduate student Ruth Foreman; and Manching Ku, a research fellow from Bradley Bernstein's lab at Massachusetts General Hospital. Konrad Hochedlinger also contributed.
Using artificial viruses called vectors, the team activated the same four genes in a batch of mouse skin cells. These genes, Oct4, Sox2, c-Myc and Klf4, are called transcription factors, meaning that they regulate large networks of other genes. While Oct4 and Sox2 are normally active in the early stages of embryogenesis, they typically shut down once an embryo has developed beyond the blastocyst stage.
"We were working with tens of thousands of cells, and we needed to devise a precise method for picking out those rare cells in which the reprogramming actually worked," says Wernig. "On average, it only works in about one out of 1,000 cells."
To test for reprogramming, the team decided to zero in on Oct4 and another transcription factor called Nanog. These two hallmarks for embryonic stem cell identity are only active in fully pluripotent cells. The trick would be to figure out a way to harvest Oct4- and Nanog-active cells from the rest of the population.
The answer came in the form of a laboratory technique called "homologous recombination." Here, the scientists took genetic material known to be resistant to the toxic drug neomycin, and spliced it into the genomes of each cell right beside Oct4 and Nanog. If Oct4 and Nanog switched on, the drug-resistant DNA would also spring into action. The researchers then added neomycin to the cells. Only those fully reprogrammed cells with an active Oct4 and Nanog survived.
Next, the team ran these cells through a battery of tests, seeing if they could discover any substantial differences between these cells and normal embryonic stem cells.
"In all tests, both genetic and epigenetic, there were no molecular markers distinguishing these two groups," says Meissner.
But definitive proof would only come through demonstrating that these cells could actually develop into any kind of body tissue and cell type. The researchers approached this question in three ways.
First, they fluorescently labeled these reprogrammed cells and injected them into early-stage embryos, which eventually gave rise to live mice. While these mice consisted of both the reprogrammed cells and the natural cells from the original embryo, the fluorescent tags indicated that the reprogrammed cells contributed to all tissue types in the mouse—everything from blood to internal organs to hair color.
Next, they bred these mice and found lineages of the reprogrammed cells in the subsequent generation, proving that these new cells had contributed to the germ line.
Finally, the team took advantage of another lab technique that involves creating a genetically abnormal embryo whose cells all consist of four chromosomes, rather than two. Because of this aberrant formation, the embryo can only form a placenta, and cannot develop into a full-term fetus. The researchers injected the reprogrammed cells into this embryo, and then implanted it in a uterus. Eventually live late-gestation fetuses could be recovered—created exclusively from the reprogrammed cells.
"This is the most stringent criteria anyone can use to determine if a cell is pluripotent," says Jaenisch.
Still, many technical hurdles remain for possibly translating this work to human cells. For example, the homologous recombination technique used to isolate the pluripotent cells does not yet work in human embryonic stem cells. Also, using cells that contain viral vectors can pose health risks.
"We are optimistic that this can one day work in human cells," says Wernig. "We just need to find new strategies to reach that goal. For now, it would simply be premature and irresponsible to claim that we no longer need eggs for embryonic stem cell research."
This research is supported by the National Institutes of Health.
****
Rudolf Jaenisch’s primary affiliation is with Whitehead Institute of Biomedical Research, where his laboratory is located and all his research is conducted. He also is a professor of biology at Massachusetts Institute of Technology.
****
Wernig, M., Meissner, A., Foreman, R., Brambrink, T., Ku, M., Hochedlinger, K., . . . Jaenisch, R. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature, 448(7151), 318-324. doi:10.1038/nature05944
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


