New role for an old protein: Cancer causer

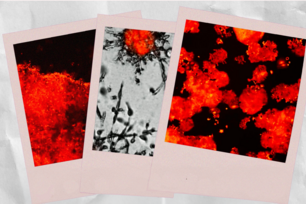
In cells that express wild-type RAB35 (left), activation of the platelet derived growth factor receptor alpha (PDGFRa) by the growth factor PDGF-AA leads to activation of PI3K/AKT signaling and internalization and trafficking of the PDGFRa. However, in cells that express a mutant version of RAB35 that is permanently activated (right), PDGFRa remains internalized in the absence of PDGF-AA and signals to PI-3 kinase (PI3K)/AKT. Investigators from the Sabatini lab at WIBR and the Sawyers lab at MSKCC think that mutant RAB35 serves to retain active PDGFRa in a late endosome or lysosome, where it can signal to downstream signaling pathways like PI3K/AKT.
CAMBRIDGE, Mass. – A protein known to play a role in transporting the molecular contents of normal cells into and out of various intracellular compartments can also turn such cells cancerous by stimulating a key growth-control pathway.
By conducting a large-scale search for regulators of the signaling pathway known as PI3K/AKT, which promotes cell survival, growth, and proliferation—and which is highly active in cancer cells—researchers at Whitehead Institute and Memorial Sloan-Kettering Cancer Center have implicated the protein RAB35 in the oncogenic process. Their findings are reported online this week in the journal Science.
“Most tumors find a way to turn this pathway on, but in many tumors and cell lines there aren't always mutations that would explain PI3K/AKT activation,” says Douglas Wheeler, a former graduate student in the lab of Whitehead Member David Sabatini and first author of the Science paper. “Here we asked whether we could find new regulators of this pathway. What we found was that wild-type RAB35 is important in signaling for the PI3K/AKT pathway, and that mutant RAB35 activates PI3K/AKT signaling, which can transform cells from a normal to a cancerous state.”
Wheeler and collaborators hit upon RAB35 via an RNA interference (RNAi) screen for genes that regulate the pathway (as measured by changes in AKT phosphorylation). While the screen identified multiple known pathway regulators, it also pointed to several genes not previously implicated. The researchers then compared these novel entrants against a database of genes with mutations found in human cancers. That sort narrowed the field from nearly 7,500 candidates at the outset to 26 regulators of interest.
An analysis of the reported cellular functions of the remaining suspects pointed to the family of so-called RAB proteins, known to regulate intracellular protein trafficking, leading the investigators to wonder whether aberrant trafficking could contribute to oncogenesis. Of this group of proteins, RAB35 emerged, in part because mutant forms have been found in human tumors. Through a series of experiments, the researchers cemented RAB35’s role, establishing that elimination of the protein suppresses AKT phosphorylation (and, therefore, PI3K pathway activity), which suggested that RAB35 is necessary to activate PI3K/AKT signaling; and that cells expressing mutated forms of RAB35 found in human cancers can activate the PI3K/AKT pathway and turn normal cells cancerous in vitro.
Wheeler concedes that although reported mutations to RAB35 in human cancers are relatively rare, they seem likely to be true “driver” mutations when they are present in tumors. Further, he emphasizes that the precise way in which mutant RAB35 contributes to oncogenesis—activating a key signaling pathway by altering the movement of certain growth factor receptors through membranes into intracellular compartments—is particularly intriguing.
“The mechanism is compelling,” says Wheeler, who is now a postdoctoral fellow at Broad Institute and the Dana-Farber Cancer Institute. “In this case, RAB35 internalizes the receptor and causes the pathway to be constitutively active. This suggests that there may be an important role in dysregulated membrane trafficking in oncogenesis.”
This work was supported by the National Institutes of Health (grants CA103866, AI47389, CA155169, CA092629, NIH Director’s New Innovator Award 1DP2CA195761-01, and the NIH Medical Scientist Training Program grant GM07739), the Starr Cancer Consortium Award, the Department of Defense Prostate Cancer Research Program via the Office of the Congressionally Directed Medical Research Programs Predoctoral Prostate Cancer Training Award PC094483, the Glenn Foundation for Medical Research, and the Pew-Stewart Scholarship for Cancer Research.
* * *
David Sabatini's primary affiliation is with Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a Howard Hughes Medical Institute investigator and a professor of biology at Massachusetts Institute of Technology.
* * *
Citation
Wheeler, D. B., Zoncu, R., Root, D. E., Sabatini, D. M., & Sawyers, C. L. (2015). Identification of an oncogenic RAB protein. Science, 350(6257), 211-217.
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu