Newly created cancer stem cells could aid breast cancer research

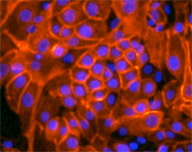
Pathologist Tan Ince transformed the normal cells into these cancerous ones (whose membranes are stained green). The transformed cells retain their sheet-forming capabilities, resembling the tumor cells found in many patients. They also possess enormous potential to create and spread tumors. As many as one in ten is a cancer stem cell.
Image: Tan Ince
CAMBRIDGE, Mass. — In some ways, certain tumors resemble bee colonies, says pathologist Tan Ince. Each cancer cell in the tumor plays a specific role, and just a fraction of the cells serve as “queens,” possessing the unique ability to maintain themselves in an unspecialized state and seed new tumors. These cells can also divide and produce the “worker” cells that form the bulk of the tumor.
These “queens” are cancer stem cells. Now the lab of Whitehead Member Robert Weinberg has created such cells in a Petri dish by isolating and transforming a particular population of cells from human breast tissue. After being injected with just 100 of these transformed cells, mice developed tumors that metastasized (spread to distant tissues).
“The operational definition of a cancer stem cell is the ability to initiate a tumor, so these are cancer stem cells,” declares Weinberg, who is also an MIT professor of biology.
Ince didn’t set out to engineer these potent cells. As a post-doctoral researcher in the Weinberg lab and gynecologic pathologist at Brigham and Women’s Hospital, he was simply trying to create breast cancer models that look like real human tumors under the microscope and behave like those seen in many patients.
|
Grown in a newly invented culture medium, these normal human breast cells (whose membranes are stained red) form sheets. Image: Tan Ince
|
In more than 90 percent of human breast tumors, cancer cells resemble those lining our body’s cavities. A trained pathologist can spot the similarities under a microscope. But the cancer cells previously engineered from normal breast cells for laboratory studies looked different. Ince suspected that researchers were transforming the wrong type of cells.
Now an independent investigator at Brigham and Women’s Hospital and an instructor at Harvard Medical School, Ince developed a recipe for a new chemically defined culture medium and managed to grow a different type of human breast cell that ordinarily dies in culture. He transformed it into a cancer cell by inserting specific genes through a standard procedure.
The engineered cells proved to be extremely powerful. When Ince injected more than 100,000 of them into a mouse with a compromised immune system, it quickly developed massive, deadly tumors. In initial experiments, a few tissue slices revealed a primary tumor structure that resembled that of cancer patients with metastases.
That prompted Ince to wonder whether the cancer cells he created would metastasize if the mouse lived longer. He repeated the experiment in other mice, reducing the number of cells in the injection to as few as 100 in hopes of slowing tumor growth. The cancer cells continued to seed tumors and those tumors metastasized. In sharp contrast, scientists must inject about 1 million cells to get a tumor when working with the cancer cell lines routinely used in the laboratory.
“In the process of making a model that reflects a tumor type common in patients, I created tumor-initiating cells,” Ince explains. “That was a complete surprise.”
“This work could provide a boon to researchers who study these elusive cancer stem cells by offering a bountiful source of them,” maintains Weinberg. “Labs can easily grow the newly created cells for use in experiments.”
The study, which appears in Cancer Cell on August 13, also offers clues about the trajectory of cancer cells. A normal cell is thought to evolve progressively toward a malignant state through a series of genetic mutations. The early alterations confer uncontrolled growth, while later alterations enable the cell to migrate and invade other tissues. Over the past decades, considerable effort has gone into discovering these tumor-initiating and metastasis-initiating genetic alterations.
The new study suggests, however, that some normal cells are more prone to become tumor-initiating cells and have a higher metastatic potential when they become cancer cells than other normal cells. The culture medium Ince created favors the growth of the human breast cells with high tumor-making and metastatic potential while the standard culture medium favors cells with low tumor-making potential. Although the two types are only slightly different, the cells behave completely differently after acquiring the same mutations.
Ince confirmed this behavioral difference by taking a single human breast tissue sample, splitting it in two and growing the cells in the two culture mediums, which select for different cells. Next, he transformed the two populations with the same tumor-initiating genes, injected them in mice and watched the result. The cells that were grown in the new culture medium were 10,000 times more potent as tumor initiators and were the only ones able to metastasize. Thus genes that were previously thought to only initiate tumors initiated metastasis, which is the main cause of cancer mortality in the clinic.
“Tan has demonstrated that a critical determinant of eventual metastasis is the identity of the normal cell type that preexists in the breast and becomes the object of mutation and selection,” Weinberg says.
This research is funded by the Breast Cancer Research Foundation and the National Institutes of Health.
****
Robert Weinberg’s primary affiliation is with Whitehead Institute of Biomedical Research, where his laboratory is located and all his research is conducted. He also is a professor of biology at Massachusetts Institute of Technology.
****
Ince, T. A., et al. (2007). Transformation of Different Human Breast Epithelial Cell Types Leads to Distinct Tumor Phenotypes. Cancer Cell, 12(2), 160-170. doi:10.1016/j.ccr.2007.06.013
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu