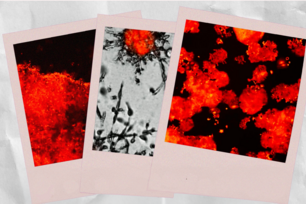
Cancer pathway exposed

This mouse embryo has been labeled with a reporter that detects the activity of the mTOR protein, stained red. (DNA is stained blue.) This image demonstrates that embryos have high levels of mTOR activity.
Image: David Guertin
CAMBRIDGE, Mass. — In the mid-1990s when Whitehead Member David Sabatini, then a graduate student at Johns Hopkins University, discovered a protein called mTOR, he had no idea that within a decade this finding would catch the attention of drug companies worldwide.
Sabatini and others had been investigating the mechanisms behind the success of rapamycin, a drug that helps prevent organ rejection in transplant patients. They found that the drug works by blocking a previously unknown protein, which was eventually dubbed mTOR (for mammalian target of rapamycin).
Scientists soon found that mTOR helps cells detect environmental nutrients and protein growth factors, which in turn influence the actual size of a cell. When rapamycin blocks mTOR, it tricks the cells responsible for organ rejection into believing that they are starving.
But scientists now realize that mTOR’s significance reaches beyond its relation to rapamycin. mTOR also plays an integral role in many cancers, including prostate and brain cancers.
Reporting in the December issue of Developmental Cell, postdoctoral scientist David Guertin and other researchers in Sabatini’s lab describe using genetic tools to show that mTOR is a critical regulator of a prominent cancer protein called AKT.
In a previous paper, then-postdoctoral researcher Dos Sarbassov, Sabatini, Guertin and colleagues showed that if proteins critical for one aspect of mTOR activity were inhibited, AKT could not activate. This implied that blocking mTOR might prevent AKT from driving tumor growth. “But that paper relied on biochemical techniques, like RNAi, to interfere with mTOR, and so not everyone in the scientific community accepted it,” says Sabatini. “In order to get the conclusive evidence that mTOR is a major player, you need to knock it out all together. And that’s what we did here.”
In the recent study, Sabatini’s lab developed mouse models in which genes necessary for this aspect of mTOR activity were deleted. Again, AKT was significantly inhibited in these animal models.
These findings strongly suggest that an inhibitor of this mTOR activity might have therapeutic value for a wide range of AKT-related tumors, such as prostate and brain cancer. Currently, many labs and drug companies are looking at various ways to block mTOR activity. (For reasons that are not clear, the original mTOR-blocking drug, rapamycin, has had limited success in treating cancer.)
“Discovering this new branch of mTOR signaling has changed how we think about mTOR’s role in cancer,” says Guertin. “This work has opened the door to new therapeutic strategies that could have a broad impact in the clinic.”
This project was funded by grants from the National Institutes of Health and by a Damon Runyon Cancer Research Foundation Fellowship.
***
Guertin, D. A., et al. (2006). Ablation in Mice of the mTORC Components raptor, rictor, or mLST8 Reveals that mTORC2 Is Required for Signaling to Akt-FOXO and PKCa, but Not S6K1. Developmental Cell, 11, 859–871.
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu