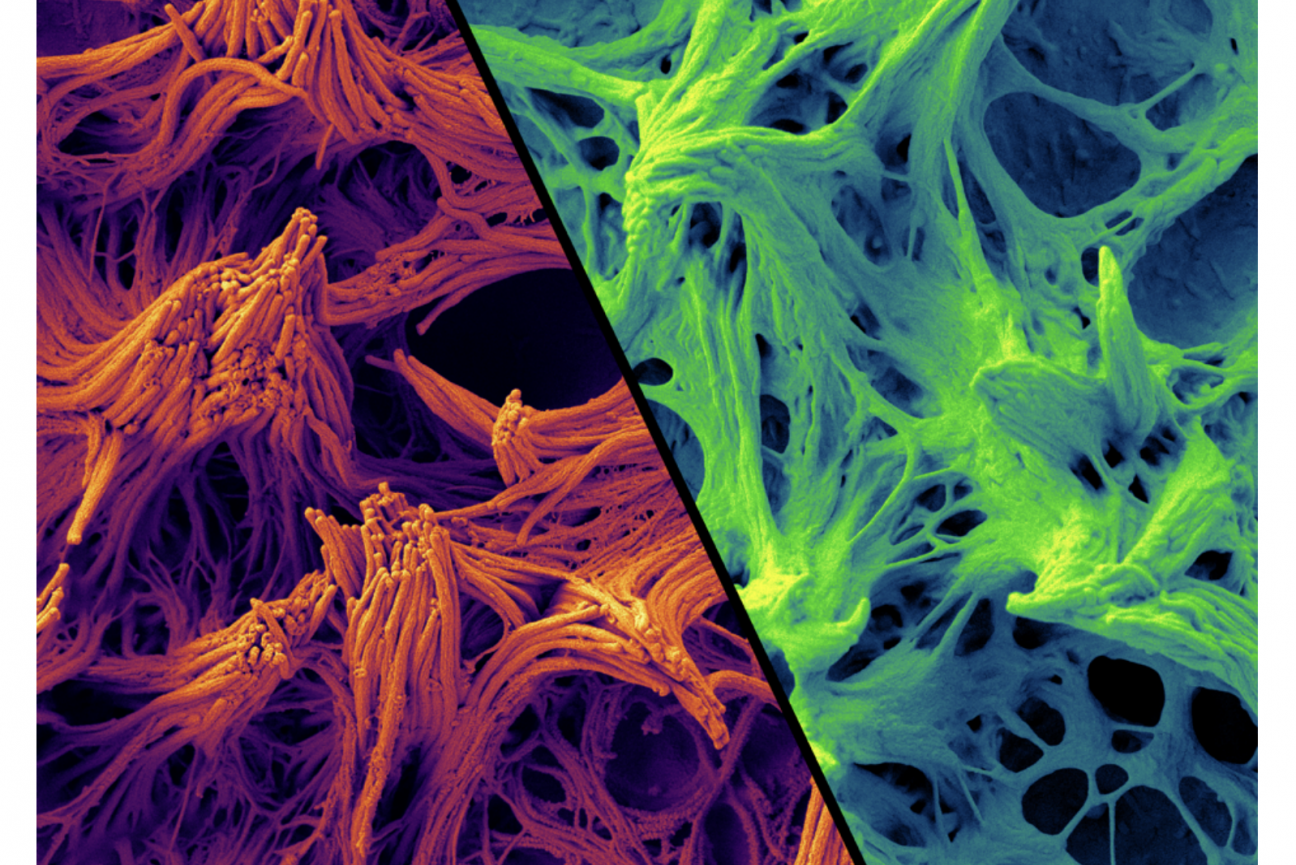
Airway cilia grown in culture. Normal cells are shown in orange, and diseased cells in green.
Raghu Chivukula
From bedside to bench, and back again
In 2018, a 31-year-old woman checked into Massachusetts General Hospital (MGH) in Boston with a respiratory infection so bad she had to be placed on oxygen. A trip to the hospital for lung trouble was nothing new for her -- several times in the past, recurrent infections required her to stay under a doctor’s supervision for days until they blew over. Now, however, it seemed that she would not be leaving the hospital until she received two entirely new lungs.
The woman had had respiratory issues since she was a baby. Her flare-ups usually presented like pneumonia -- a nasty, phlegm-y cough accompanied by a fever. After years of this the pathways between the trachea and the alveoli, called bronchi, were swollen and inflamed. Her physicians suspected that these frequent respiratory bouts had something to do with the mucus produced in her airways.
Mucus is the body’s first line of defense against the dirt and pathogens we inhale when we breathe. The sticky substance, composed mostly of water, salts, and sugar-laden proteins called mucins, traps the incoming material on its sticky surface. From there, cilia -- tiny finger-like protrusions from cells that can look like small eyelashes -- push the mucus up through the airways where it is eventually swallowed or coughed out.
Conditions such as cystic fibrosis can cause the mucus that lines the lung pathways to become so thick that the cilia can’t push it out, leading to bronchiectasis -- the swelling of the bronchi. When physicians tested the woman for such likely causes, however, the results came back negative. Her case was a total mystery.
Cracking the case study
As she awaited her double lung transplant, the woman met Dr. Raghu Chivukula, at the time a pulmonary and critical care medicine fellow at MGH interested in rare and unusual lung diseases as a consequence of his PhD training in human genetics. During his time spent working with these often critically ill patients, “it became clear that there were lots of unanswered questions in lung biology and the basis of lung diseases,” he said. Chivukula soon realized that the woman’s condition was one of these unanswered questions.
Often, when doctors are unable to come to a diagnosis, they end up referring a patient to another hospital or to see a specialist. MGH, with its reputation as one of the top hospitals in America, sees quite a lot of these mysterious cases. They saw so many, in fact, that in 2016 the hospital created a program called the Pathways Consult Service, where scientists could evaluate these unusual patients to see whether their maladies might be something entirely new to science. The program helps connect physicians with researchers in the Boston area to help come up with the technology and resources to dive deep into the biology of the patients’ undiagnosed conditions.
After his initial conversations with the woman with the lung condition, Chivukula reached out to the Pathways program to see whether they could help him further investigate her disease.
“We were so excited when Raghu, who is an incredible physician and scientist, came to us with this opportunity to learn about biology from this patient that he was seeing,” says Dr. Katrina Armstrong, the Physician-in-Chief of the Department of Medicine at MGH who works with the Pathways program.
As the woman waited for her lung transplant, Chivukula interviewed her about her medical history. He also talked to two of her siblings, who were in town to help their sister in the run-up to her operation. Talking to the three of them offered Chivukula a clue: respiratory infections ran in the woman’s family. Her two siblings showed similar, if milder, symptoms.
This finding led Chivukula, with help from the Pathways program, to send the genetic material of the woman, her parents, and her two siblings to Fowzan S. Alkuraya, a geneticist at King Faisal Specialist Hospital and Research Centre (KFSHRC), in Riyadh, Saudi Arabia. When the results came in, Alkuraya sifted through the data looking for mutations that could be playing a role in the family’s lung issues. Across all three genomes, one common difference stood out: a mutation in a gene called NEK10. “I wrote back to Raghu to tell him how excited I was for having identified this novel gene,” Alkuraya says.
Scientists weren’t sure what this gene did, although they knew it coded for a kinase -- a type of protein involved in signalling by modifying other proteins with a phosphate group. Previous studies suggested the NEK10 protein might play a role in how cancer cells respond to DNA damage in humans and the formation of the nervous system in certain kinds of fish, but no research had ever linked its activity to any kind of human disease, or to the respiratory system.
Once he realized the woman’s mutation was affecting a kinase, Chivukula decided to take on the project as part of his postdoctoral research in David Sabatini’s lab at Whitehead Institute. Chivukula had initially begun working with Sabatini on a project about the role of lysosomes in the development of pulmonary fibrosis. Since Sabatini’s previous research has included a focus on understanding important protein kinases in cells, the new mutation seemed like a perfect additional project. “I was hopeful that the combination of my own interests in lung biology with David’s lab’s world-class cell biology expertise and specialized toolkit would allow us to figure out this disease,” Chivukula says.
The mystery mutation
To determine whether this mutation could be to blame for the woman’s condition, Chivukula and Sabatini took a closer look at the mutation itself; the changes in the woman’s DNA sequence didn’t make her cells express less NEK10, they found. Instead, the alteration caused the insertion of 7 additional amino acids in the NEK10 protein, which Chivukula hypothesized might render the protein unstable and not able to perform some key job in the woman’s lung cells.
Still, she was only one patient, and it was possible this specific mutation that appeared in the DNA of her and her siblings was unrelated to her condition. Was this just a fluke, the scientists wondered, or could NEK10 mutations be to blame in other cases of unexplained respiratory problems?
Chivukula started sending out feelers to other hospitals and research centers around the world. He hoped to find other patients with unexplained lung conditions that shared the mutations the woman and her siblings had in their NEK10 genes. Slowly, other accounts trickled in. Other hospitals had registered similar changes in patients’ DNA coding for the NEK10 protein, but didn’t have enough evidence to tie the gene to their conditions.
Chivukula’s search eventually turned up six additional patients. All of them -- including several under the age of 25 -- had different mutations in the NEK10 gene, but overall the effects were the same: changes in the amino acid sequence of the NEK10 protein, and a condition similar to the woman’s, marked by pneumonia-like flares and swollen, enlarged airways. Whatever NEK10 was doing, the scientists could now assume it was associated with keeping the pathways to and from the lungs healthy.
Armed with the evidence that this mutation was associated with these patients’ conditions, Chivukula went back to the lab to find out what exactly NEK10 was doing in cells. First, he needed to find where it was being used. To do this, he turned to mRNA, or messenger RNA, the intermediate step between DNA and proteins. When a cell needs to express a certain gene, it creates an mRNA transcript. That transcript carries the genetic information to the ribosomes, where it is made into a protein.
Chivukula and his colleagues obtained airway tissue from the woman -- her transplant meant they had good access to tissues to study -- as well as from a few from people with normal lungs. They used a kind of genetic testing that allowed them to see what RNA was being expressed in the cells, offering a clue to where the protein was used: there were large quantities of NEK10 mRNA in specialized airway tissue, but hardly any in undifferentiated lung stem cells.
To see if they could induce these undifferentiated cells to produce NEK10, the researchers cultured them in the laboratory, using a trick to mimic the lining of a human airway. By allowing the stem cells to grow on a thin film where liquid medium meets the air, the researchers coaxed the cells to slowly mature and differentiate into airway cells in the lab. When the researchers looked at this lab-grown tissue carefully, they found much higher expression of NEK10 mRNA. This meant that whatever the protein was doing, it was most active in the cells that lined the airways.
Next they wondered whether the protein might be functioning specifically within one type of airway cell, of which there are many varieties with different roles. To test this, they used a fluorescent protein to mark the cells expressing NEK10, making these cells glow green. When they allowed the cells to differentiate, the brightest glowing cells were those that were covered in cilia. This suggested to the researchers that the woman’s condition was a kind of ciliopathy, or disorder associated with cilia. Nearly all vertebrate cells have some kind of cilia, and mutations that affect their structures can have consequences such as polycystic kidney disease, retinal disease, and conditions such as obesity and cerebral anomalies.
In the lungs, cilia move mucus by wiggling back and forth in tandem with their neighbors. Moreover, previous studies had found that disruption of airway cilia could cause a disease akin to that seen in NEK10 patients. When Chivukula took a closer look at the woman’s airway cilia, he found that they still wiggled at the same speed, but something was off; while normal cilia could transport polystyrene beads on a slithery wave of mucus, her mutated cilia could barely move mucus at all.
Under a microscope, the cilia were strangely clumpy and underdeveloped. The mutation, it turned out, had caused the cilia to be too short to effectively move mucus, leading to a build-up in her airways. This mucus build-up increased her likelihood of respiratory infections and, with each infection, her bronchi grew more enlarged and swollen until she could barely breathe on her own.
A new disease
Chivukula, Sabatini, and coauthors published their findings on the new disease in Nature Medicine in February. From what they’ve observed in the seven patients they studied, the condition follows an autosomal recessive inheritance pattern -- the gene must be knocked out in both copies for airway cilia to be affected -- much like cystic fibrosis and most forms of ciliopathy that affect the lungs or other tissues.
Further research will determine how variable the condition can be depending on the type of mutation in the NEK10 gene. “It's entirely possible that there are milder or subtler variants of this gene that are not, on their own, causing this sort of end-stage lung disease,” Chivukula says.
That might mean a mutation in the NEK10 gene that led to a protein that was deformed rather than completely unstable, he says, although at this point it is impossible to know for sure. “What we do know is that this double knockout of the gene sort of phenotype is quite rare,” he says. “But like for many genetic diseases, once you understand the severe ones, you can use that information to really dig into the more common forms.”
As for the woman who received the double lung transplant, “She's doing pretty well,” says Chivukula. “She doesn't need oxygen and can finally walk around without becoming short of breath. Being sick for 20 years takes its toll like it would for anyone, but she's in a much better state than she was before her transplant.”
Dr. Armstrong and others at MGH are excited by the potential applications of Chivukula’s findings. “It's pretty unusual [for a Pathways case] to have quite as beautiful a story as Raghu was able to put together that quickly,” she says.
Maybe in the future, Chivukula says, other patients in the woman’s position will be able to be treated before their condition becomes severe enough to need a transplant in the first place. Although much research remains to be done before the condition could be cured, Chivukula believes the potential is there. Cilia, he points out, have been shown to change slightly due to external causes. For example, smokers can have cilia that are a tiny bit shorter than those of non-smokers.
“We've shown that delivering extra active NEK10 protein actually causes cilia function to be improved, so that does suggest that this condition could be druggable in the future,” he says. “We just need to understand the biology a little bit better.”
Chivukula, R. et al. A human ciliopathy reveals essential functions for NEK10 in airway mucociliary clearance. Nature Medicine. 2020 Feb. doi: 10.1038/s41591-019-0730-x.
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


