Three-banded panther worm debuts as a new model in the study of regeneration
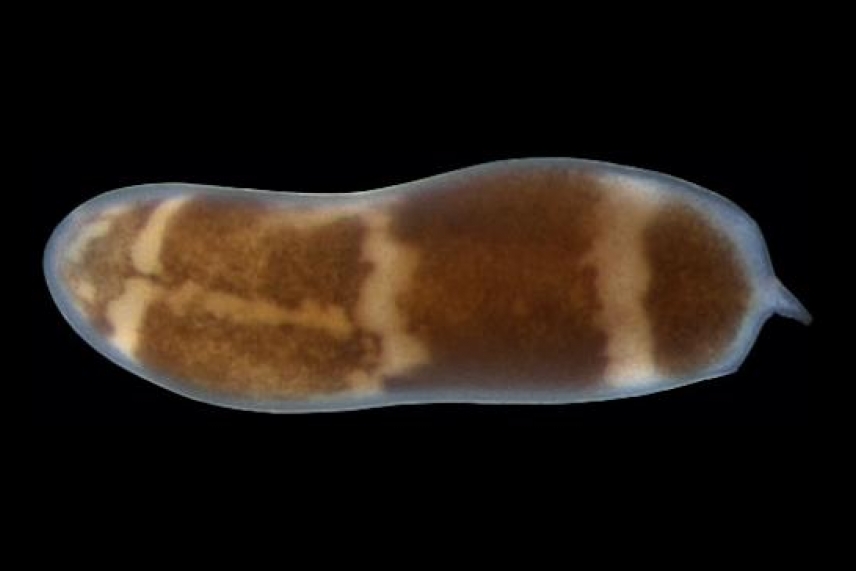
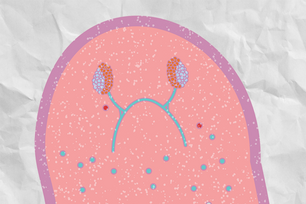
The lab of Whitehead Institute Member Peter Reddien is introducing the scientific community to the three-banded panther worm (Hofstenia miamia, left), a small organism with the ability to regenerate any missing body part. As a model, Hofstenia could help further our understanding of regeneration, how its mechanisms have evolved over millennia, and what limits regeneration in other animals, including humans. Intriguingly, Hofstenia and the planarian Schmidtea mediterranea—long the mainstay of Reddien’s research—rely on similar molecular pathways to control regeneration despite having evolved separately over the course of roughly 550 million years.
Kathleen Mazza-Curll/Mansi Srivastava/Whitehead Institute
CAMBRIDGE, Mass. (April 24, 2014) – Closely resembling plump grains of wild rice set in motion, the three-banded panther worms swimming in disposable containers in Whitehead Institute Member Peter Reddien’s lab hardly seem like the next big thing in regeneration. And yet, these little-studied organisms possess the ability to regenerate any part of their bodies and are amenable to molecular studies in the lab, making them a valuable addition to a field keen on understanding how mechanisms controlling regeneration have evolved over millennia and how they might be activated in humans.
Four years ago, postdoctoral researcher Mansi Srivastava and Reddien, collected these intriguing animals swimming among submerged mangrove leaves and other aquatic detritus in a chilly Bermudan pond. Known scientifically as Hofstenia miamia, the worm earned its common name from the three cream-colored stripes running across its body as well as its voracious appetite for live prey. Found in the Caribbean, Bahamas, Bermuda, and even as far away as Japan and the Red Sea, the worms were reported to be endowed with regenerative capabilities. In the 1960s, one scientist described the fact that three-banded panther worms could regrow a severed head, although no additional reports followed.
“It was a big risk for us—it was not a project where you knew it was going to work from the beginning,” says Reddien, who is also an associate professor of biology at MIT and a Howard Hughes Medical Institute (HHMI) Investigator. “I had no idea how successful we’d be with culturing the animals or how successful the methods for development would be. There are all kinds of ways we could’ve failed on this one, but it was fun. It’s the kind of science that has an adventurous spirit to it. And the organism is an even better model organism than we could have hoped for.”
Reddien and Srivastava present their new model to the scientific community in the May 19th issue of the journal Current Biology.
Once the worms arrived in their new home in Cambridge, the first test was to acclimate them to lab life and determine their needs for survival. Initially, the worms were dying. The salinity of their water was off, even though it matched the pond where they were found. Hofstenia also rejected the liver that is the dietary mainstay for Reddien’s other model of regeneration, the planarian (Schmidtea mediterranea). The three-banded panther worms shrank in size and some resorted to cannibalism.
Eventually, the water quality was fixed and a preferred food source was identified: sea monkeys, also known as brine shrimp. Now the worms are thriving and laying numerous eggs, enough to create an ample supply of animals for experiments.
Through a series of dissections, Reddien and Srivastava established that Hofstenia not only regenerate their heads, but, like planarians, are also able to regrow any body part. The scientists then documented the worm’s transcriptome—a list of all of the genes that are transcribed in the animal—and established that RNA interference (RNAi) could be used in this animal to inhibit specific genes and unlock the molecular functions that allow regeneration.
With these tools in hand, they determined that in Hofstenia, as in planarians, Wnt signaling controls regeneration along the anterior-posterior (head-tail) axis and Bmp-Admp signaling controls regeneration along the dorsal-ventral (back-belly) axis.
If Hofstenia and planarians were phylogenetically close, such similarities would not be surprising. But after analyzing the Hofstenia transcriptome, the team determined that the three-banded panther worm and planarians are only very distantly related, a view that had been proposed based on analyses with sequences from a small number of genes.
“I find that there is no evidence, even with this large dataset, for Hofstenia to be classified with planarians, which means the last common ancestor that these two species shared existed 550 million years ago. This is the common ancestor that we, humans, also share with these species,” says Srivastava, who has a background in evolutionary developmental biology and authored the Current Biology article. “The cool thing is that this raises the question of whether our common ancestor used these pathways—Wnt and Bmp signaling—to regenerate or not.”
This work is supported by the Jane Coffin Childs Memorial Fund, Human Frontier Science Program, and the Keck Foundation.
* * *
Peter Reddien’s primary affiliation is with Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a Howard Hughes Medical Institute Investigator and an Associate Professor of Biology at the Massachusetts Institute of Technology.
* * *
Citation
Srivastava, M., Mazza-Curll, K. L., van Wolfswinkel, J. C., & Reddien, P. W. (2014). Whole-body acoel regeneration is controlled by Wnt and Bmp-Admp signaling. Current biology, 24(10), 1107-1113.
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu