Flu virus wipes out immune system’s first responders to establish infection
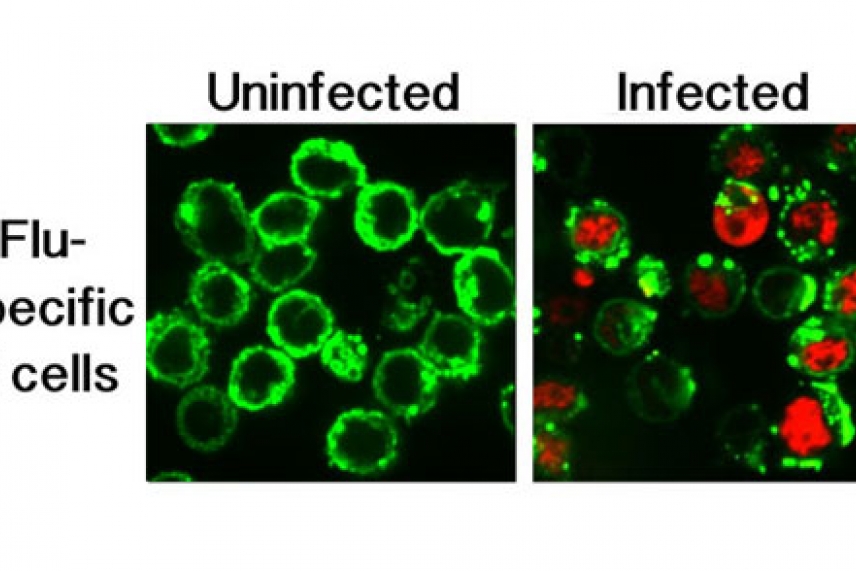
Uninfected B cells and B cells infected with influenza
Courtesy of Nature
CAMBRIDGE, Mass. – Revealing influenza’s truly insidious nature, Whitehead Institute scientists have discovered that the virus is able to infect its host by first killing off the cells of the immune system that are actually best equipped to neutralize the virus.
Confronted with a harmful virus, the immune system works to generate cells capable of producing antibodies perfectly suited to bind and disarm the hostile invader. These virus-specific B cells proliferate, secreting the antibodies that slow and eventually eradicate the virus. A population of these cells retains the information needed to neutralize the virus and takes up residence in the lung to ward off secondary infection from re-exposure to the virus via inhalation.
On the surface of these so-called memory B cells are high-affinity virus-specific receptors that bind virus particles to reduce viral spread. While such cells should serve at the body’s first line of defense, it turns out that flu virus exploits the specificity of the cells’ receptors, using them to gain entry, disrupt antibody production, and ultimately kill the cells. By dispatching its enemies in this fashion, the virus is able to replicate efficiently before the immune system can mount a second wave of defense. This seemingly counter-intuitive pathway to infection is described this week in the journal Nature.
“We can now add this to the growing list of ways that the flu virus has to establish infection,” says Joseph Ashour, a co-author of the Nature paper and a postdoctoral researcher in the lab of Whitehead Member Hidde Ploegh.
“This is how the virus gains a foothold,” adds Ploegh lab postdoc Stephanie Dougan, also a co-author of the study. “The virus targets memory cells in the lung, which allows infection to be established—even if the immune system has seen this flu before.”
Discovering this dynamic of the virus was no small task, in part because virus-specific B cells are found in exceedingly small numbers and are extremely difficult to isolate. To overcome these challenges, Dougan together with students Max Popp and Roos Karssemeijer leveraged a protein-labeling technology developed earlier in the Ploegh lab to attach a fluorescent label to influenza virus, thus identifying flu-specific B cells by their interaction with fluorescent flu micelles. This step was essential because no flu protein can be tagged in the conventional manner with green fluorescent protein (GFP) in the context of an infectious virus. Dougan then introduced the B cells’ nuclei into enucleated mouse egg cells via somatic cell nuclear transfer (SCNT)—a cloning technique she learned in Whitehead Founding Member Rudolf Jaenisch’s lab—to generate a line of mice with virus-specific B cells and cell receptors.
Though complicated, the generation of mice with B cells specific for a known pathogen allowed Dougan and Ashour to track the virus’s interactions with the cells in unprecedented fashion. Because the infectious process they discovered is likely not exclusive to influenza virus, these scientists believe their approach could have implications for other viruses as well.
“We can now make highly effective immunological models for a variety of pathogens,” says Dougan. “This is actually a perfect model for studying memory immune cells.”
Adds Ashour: “This is research that could help with rational vaccine design, leading to more effective vaccines for seasonal flu. It might even suggest novel strategies for conferring immunity.”
This work is supported by the Cancer Research Institute, the Pancreatic Cancer Action Network, the Human Frontiers Science Program, and the National Institutes of Health.
***
Hidde Ploegh’s primary affiliation is with Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a professor of biology at Massachusetts Institute of Technology.
***
Citation:
Dougan, S. K., Ashour, J., Karssemeijer, R. A., Popp, M. W., Avalos, A. M., Barisa, M., ... & Alt, F. W. (2013). Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature, 503(7476), 406-409.
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu

