Reprogrammed cells reduce Parkinson's symptoms in rats
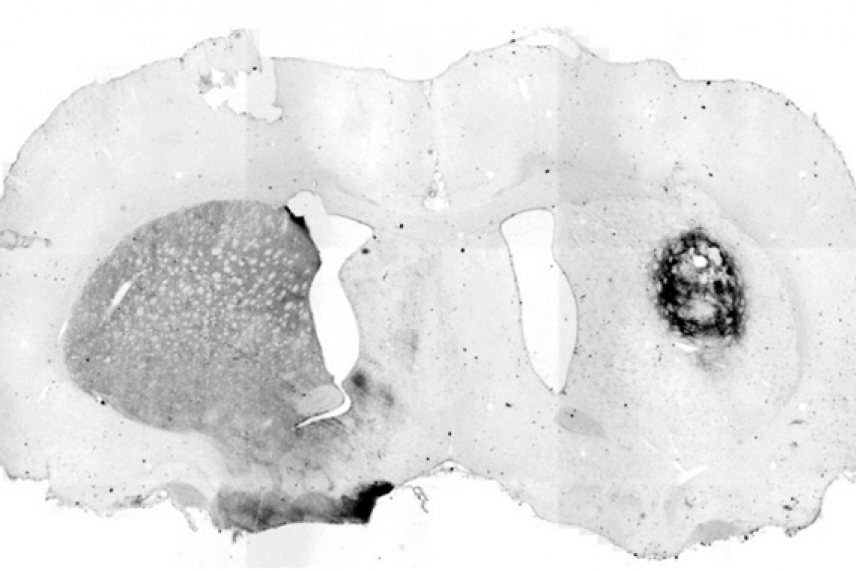
In rats that model Parkinson's disease, transplanted dopamine neurons created from IPS cells show evidence of producing dopamine precursor cells, as shown by the darker stained area on the right side. The left half of this brain shows normal production of dopamine precursors.
Image: Marius Wernig
CAMBRIDGE, Mass. - Neurons derived from reprogrammed adult skin cells successfully integrated into fetal mouse brains and reduced symptoms in a Parkinson's disease rat model, according to a study published on April 7 in the online Early Edition of PNAS.
"This is the first demonstration that reprogrammed cells can integrate into the neural system or positively affect neurodegenerative disease," says Marius Wernig, lead author of the article and a postdoctoral researcher in Whitehead Member Rudolf Jaenisch's lab.
Researchers in the Jaenisch lab showed in December 2007 that mice with a human sickle-cell anemia disease trait could also be treated successfully with adult skin cells that had been reprogrammed to an embryonic stem cell-like state.
|
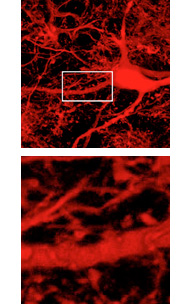
Top, neural cells derived from induced pluripotent stem cells, transplanted into embryonic mice, matured in the brain and show synaptic integration into surrounding host neurons. Bottom, a close-up view. Image: Marius Wernig
|
For the neural experiments Wernig used induced pluripotent stem cells (iPS cells), which were created by reprogramming adult skin cells using retroviruses to express four genes (Oct4, Sox2, c-Myc and Klf4) into the cells' DNA. The iPS cells were then differentiated into neural precursor cells and dopamine neurons using techniques originally developed in embryonic stem cells.
In one experiment, Wernig transplanted the neural precursor cells into brain cavities of mouse embryos. The mice were naturally delivered and analyzed nine weeks after the transplantation. Wernig saw that transplanted cells formed clusters where they had been injected and then migrated extensively into the surrounding brain tissues. Using electrophysiological studies conducted by Martha Constantine-Paton from MIT's McGovern Institute for Brain Research and structural analysis, Wernig also saw that the neural precursor cells that migrated had differentiated into several subtypes of neural cells, including neurons and glia, and had functionally integrated into the brain.
In another study, Wernig used a rat model for Parkinson's disease, a human condition caused by insufficient levels of the hormone dopamine in a specific part of the midbrain. To mimic this state, the dopamine-producing neurons were killed on one side of the rat brains. In collaboration with Ole Isacson's group at McLean Hospital/ Harvard Medical School, Wernig then grafted differentiated dopamine neurons into a part of the rat brains called the striatum.
Four weeks after surgery, the rats were tested for dopamine-related behavior. In response to amphetamine injections, rats typically walk in circles toward the side with less dopamine activity in the brain. Eight of nine rats that received the dopamine neuron transplants showed markedly less or even no circling. Eight weeks after transplantation, Wernig could see that the dopamine neurons had extended into the surrounding brain.
"This experiment shows that in vitro reprogrammed cells can in principle be used to treat Parkinson's disease," says Jaenisch. "It's a proof of principle experiment that argues, yes, these cells may have the therapeutic promise that people ascribe to them."
Jaenisch and Wernig are optimistic that this work eventually could be applied to human patients, but caution that major hurdles must be addressed first. Those include finding alternatives to the potentially cancer-causing retroviruses used to transform the skin cells into iPS cells and figuring out the best methods and places to transplant the neural precursor cells in humans.
The research was supported by the Ellison Medical Foundation and the National Institutes of Health.
* * *
Rudolf Jaenisch's primary affiliation is with Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a professor of biology at Massachusetts Institute of Technology.
* * *
Wernig, M., Zhao, J., Pruszak, J., Hedlund, E., Fu, D., Soldner, F., . . . Jaenisch, R. (2008). Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proceedings of the National Academy of Sciences, 105(15), 5856-5861. doi:10.1073/pnas.0801677105
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu