
Meet the Scientists Keeping Whitehead Institute’s Core Facilities Thriving
Behind the scenes of each new discovery made at Whitehead Institute is a committed group of scientists who keep the Institute’s core facilities running. The cores enable the Institute’s researchers to get the data they need, to frame their questions in the most insightful ways, and to take advantage of the latest technologies in each field. Meet some of these remarkable scientists here in our series, #ThankYouCoreFacilities.
Heather Keys
Functional Genomics Platform
 |
| Functional Genomics Platform Manager Heather Keys Credit: Conor Gearin/Whitehead Institute |
The gene editing system CRISPR-Cas9 is transforming the way biology works. One of the most exciting uses goes far beyond modifying a single stretch of DNA — instead screening the entire genome for genes that play a role in a particular phenotype or a disease. At Whitehead Institute, Heather Keys manages the Functional Genomics Platform, which aids scientists in making use of this new tool and adapting CRISPR screens to new research settings.
“CRISPR screens are the new big thing,” Keys says. “It opens up a lot of doors for different types of science.”
Keys joined the platform in 2016, not long after it was created. She was completing her PhD in Whitehead Institute Member David Sabatini’s lab and was planning to enter industry, but the chance to help develop a platform centered around CRISPR screens was too good to pass up.
Keys explains that the simplest type of CRISPR screen involves taking a large collection of cells and knocking out a single gene in each one using a library of guide RNAs that tell the Cas9 enzyme where to cut. Using multiple guide RNAs for the same gene helps mitigate any off-target effects that an individual guide RNA may produce. The cells are maintained for several weeks or even months, passaged many times to new culture media in order to see which cell populations thrive and which dwindle away. At various stages during this process, Keys and her team may handle more than a billion cells when screening the full human genome. The number of cells in each cell population that had a particular gene targeted tells the researchers which genes were necessary for cell growth and survival.
Now that the Functional Genomics Platform has become adept at this straightforward assay, Keys is helping researchers adapt CRISPR screens to new types of questions. For example, rather than seeing which genes are needed for cells to grow, the platform can test for genes that sensitize cancer cells to chemotherapy treatments. Genes can be up- or down-regulated rather than knocked out. Keys is also working with Whitehead Institute Fellow Kristin Knouse to create a CRISPR screen that could be used in the livers of live mice. “Hopefully that will be coming soon, and hopefully we’ll be able to make that more accessible to other people,” she says.
Keys encourages scientists to get in touch if they ever think a CRISPR screen might help them with a research question. “We’re very busy, but we’re always able to put someone in the queue,” she says. “We’re always happy to chat and talk about whether a screen would be right for their question.”
Adapting CRISPR screens to new contexts is a rewarding challenge, Keys says. “The environment is so creative here — I never know who’s going to email me next and say, hey, I want to screen this phenotype,” she says. “You’re always surprised by what’s coming down the pipeline next, and it’s really cool to be a part of that. Everybody does such high-quality work, everybody has such interesting ideas.”
In her free time, Keys likes to hang out on her boat. She lives in Quincy, and her boyfriend has been a boater for many years. The motorboat takes a lot of work and maintenance. “Now that things are going well with it, we’re trying to enjoy it and get out as much as we can,” she says. The vessel lets them reach the less accessible Boston harbor islands, as well as further destinations like Gloucester and Portsmouth. “But sometimes it’s just about hanging out at the marina — the boating life,” she says.
George Bell
Bioinformatics and Research Computing (BaRC)
 |
| Bioinformatics and Research Computing (BaRC) Director George Bell Credit: Conor Gearin/Whitehead Institute |
At the end of his PhD research in chicken embryo development, George Bell realized that it was time for a change. “I happened to have a quite repetitive project,” he says. “I kind of had enough of that. One of the advantages of bioinformatics is that if you have to do the same thing over and over, you just write a program to automatically do it lots and lots of times. You have very little time to get bored.” Over time, he became more interested in the computational techniques he was using than the lab work itself.
The move to working in bioinformatics full time made sense for the job market as well as his interests, Bell says. In the early 2000s, sequencing of the human genome was opening up opportunities to look at all genes at once, and making sense of the genome required lots of computational methods. By now, bioinformatics has become mainstream, and virtually every molecular biologist can take advantage of genomics and bioinformatics at some point during their research.
“There’s also a huge demand for people who can share their expertise across multiple labs,” Bell says. “Every professor doesn’t have to have their bioinformatics person that they might just need from time to time.”
As BaRC director at Whitehead Institute, Bell gets to collaborate with many different researchers. “Being a staff scientist in a core group is a fantastic position, because you get to do science all day every day, and you get to jump around to lots and lots of different projects,” he says.
BaRC works with researchers where they are, meaning that the team can train people in computational methods and also can run complete analyses if need be. The team also helps maintain and update software that is crucial for particular labs in the long term. The alternative would be laborious handoffs between each successive generation of students and postdocs.
“Someone could figure out any of their bioinformatics needs on their own,” Bell says. “However, we’ve probably already made every mistake they’re going to make, and we’ve already figured out how to solve most of them.”
Computational techniques keep becoming more important, Bell says. “People’s experiments are getting bigger, more complex, and more quantitative,” he says. “The combination of those three things mean that people need more complex computer programs and more statistics to answer a lot of the questions.” For those needs, BaRC is there to help, he says.
Bell says all the interesting computational challenges that researchers bring to BaRC are what make him excited to come into work every morning. Outside of work, Bell spends lots of time with his family and loves to explore Arlington's Spy Pond with his four children, whether swimming, paddle boarding, kayaking, or ice skating.
Caroline Lewis
Metabolite Profiling Core
 |
| Metabolite Profiling Core Director Caroline Lewis Credit: Conor Gearin/Whitehead Institute |
Caroline Lewis, director of the Metabolite Profiling Core Facility, is excited about the core’s new instrument. Her team helps Whitehead Institute researchers study chemicals produced in cellular reactions, known as metabolites. These compounds greatly outnumber larger molecules in the cell such as proteins — and many of them have not even been identified.
“In metabolomics, there’s a lot of excitement around untargeted metabolomics,” Lewis says. “The instrument we now have should help with that — it’s been designed specifically for searching for and identifying unknown compounds.”
The device has an impressive name — Orbitrap ID-X Tribrid Mass Spectrometer — but the important thing is that it brings a new layer to the analyses that the core can perform. Lewis explains that once you know the retention time and mass-to-charge ratio of your metabolite, you still might need more information to confirm the identity of the metabolites present. The next step is to fragment the compounds and examine the fragmentation pattern, comparing it to databases.
“What the new instrument does is fragment those fragments,” says Lewis. “It also allows us to collect fragmentation data on very low-abundance species.” The device remembers which 10 compounds it fragmented on the previous step and moves on to the next most abundant 10 compounds. This highly refined procedure lets researchers search for potentially important but less concentrated compounds.
Using liquid chromatography and gas chromatography mass spectrometry (LCMS and GCMS), Lewis and her team help Institute researchers better understand what’s happening in their cells. Even if scientists know an enzyme catalyzes a particular reaction, just measuring the levels of the enzyme itself might not give the full picture, Lewis says. “In fact, metabolic enzymes are often very highly expressed, so expression changes don’t necessarily equal changes in a metabolic reaction,” she says. “The ability to actually measure these metabolites gives you more insight into what’s really going on.”
When a researcher approaches Lewis interested in metabolomics, the first step is talking through the best approach. “They come to us and we have a discussion about what question they really want to answer,” she says. “Then we can think about what would be the best experiment, and which method would help answer that question.”
This process of collaborating and providing guidance is what Lewis enjoys the most about her role. “I enjoy talking to other people about their projects, because I’ve worked on experiments I never would have done, if I had just been running my own research group,” she says. “I’ve worked on zebrafish, the nematode Caenorhabditis elegans (C. elegans), yeast, mouse tissues, tumors, and all kinds of different cell lines. People are doing some really exciting science here, and it’s great to be a part of it.”
Lewis came to Whitehead Institute after her postdoctoral research across the street at the Koch Institute. “I decided that I didn’t necessarily want to be faculty myself, and that actually what I enjoyed most about the postdoc experience was thinking about the science, analyzing data, and interpreting data,” she says.
However, Lewis’s expertise from her research experiences, particularly in stable isotope tracing, have helped her shape the development of the core. “You can use your past knowledge, your PhD and postdoc experiences, to think about what new technology should we bring to Whitehead Institute, and how can we apply this to the members’ research programs,” she says.
Outside of the Institute, Lewis sings in the Park Street Church Choir as an alto, and particularly enjoys singing works by John Rutter or Felix Mendelssohn. She likes being near the ocean in Massachusetts, as she grew up in the United Kingdom, where the water is never more than about two hours away. She enjoys paddle-boarding and kayaking, as well as hiking in the White Mountains of New Hampshire.
Tom Volkert
Genome Technology Core
 |
| Genome Technology Core Director Tom Volkert Credit: Conor Gearin/Whitehead Institute |
Genome Technology Core Director Tom Volkert arrived at Whitehead Institute Member Richard Young’s lab in 1999 during a transitional time for biological research. The human genome project was nearing completion and there was a distinct trend toward high-throughput and big-data biology.
“It was an interesting and exciting time,” Volkert says. “We had these new techniques like DNA microarrays that were enabling genome scale experiments in a single assay for the first time. Companies were racing to put their stakes in the ground for what would be the next generation of genomics research. I was fortunate that I came up in the world in a time when there was a huge explosion in the technology. Everything was just bigger, better, faster, more. I just caught onto that wave and rode it.”
Starting with DNA microarrays, and eventually evolving into next-generation sequencing, Volkert embraced the role of supporting cutting edge technologies. Eventually it was decided that the Institute needed a core facility to support all the different labs that wanted access to these new technologies and the Genome Technology Core was born.
“Genomics and transcription are fundamental to just about any biological question you’re going to ask,” he says. “Knowing which genes are on and off, upregulated or downregulated in different conditions, is absolutely fundamental to any aspect of biology.”
Volkert says that while few people start out planning to manage a core facility, staff scientist positions can be great opportunities. “It’s an excellent career path for people who find themselves looking for an alternative to the traditional academic career track,” he says. “They can still take the skills they’ve cultivated and make a very good career out of it. All these biotech companies, pharma companies, and academics need people to run these facilities.”
Twenty years after he arrived at the Institute, sequencing technologies are still in the exponential phase of their growth, Volkert says. He estimates that the upgrade cycle for next-generation DNA sequencers is only 18-24 months. “The exciting part of it from my perspective is being on the cutting edge, seeing all these changes, and being part of them,” he says. Over the years, he has evolved from a microarray specialist, to a next-generation sequencing specialist, and now is becoming adept at single-cell sequencing techniques.
Volkert says this range of specialties is a sometimes-overlooked part of being a core facilities scientist. “You don’t have to be locked into any one technology,” he says. “Keep your eyes open, there’s always something new coming over the horizon.” He also enjoys managing the budget and operations for the Genome Technology Core. “Maybe some people aren’t as interested in that aspect of it, but I happen to have fun running my own little business,” he says.
What’s more, the scope of research projects at Whitehead Institute is immense. “It is so diverse, the different ways that people are using what is ostensibly the same technology, but being used in completely different ways,” Volkert says. “It’s unique for a fairly small research institute to have such a huge diversity of research.”
In his free time Volkert likes to play a variety of sports. He’s the captain of the Whitehead Institute “Biohazards” softball team — four-time champions in the MIT intramural league — and organizes the annual retreat golf tournament.
Uma Tejaswi
Glasswashing Core
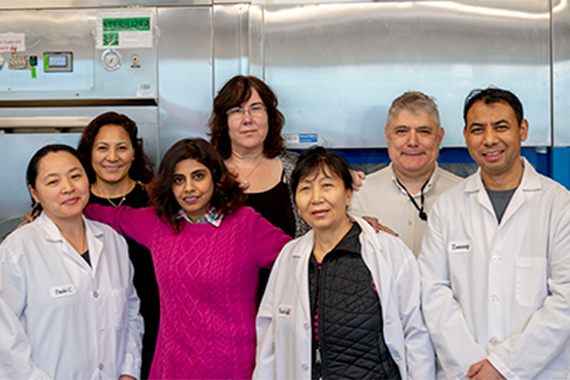 |
| Glasswashing Core Facility team Credit: Conor Gearin/Whitehead Institute |
Glasswashing Core Supervisor Uma Tejaswi says that most of her team clocks in long before sunrise. Arriving at Whitehead Institute at 4 A.M. or earlier lets the glasswashing group make sure that the hallway shelves are stocked with sterile glassware by the time that researchers arrive to begin their days. “I consider glasswashing the backbone of the Institute,” Tejaswi says. “If the researchers get in around 9 and the shelves are empty, then that stops all lab work.”
What’s more, each lab requires specialized service. The glasswashers are able to make that happen because of their long relationships with the labs here. “The same person has been working with the same lab for perhaps 18 years,” Tejaswi says. “You can blindfold them and they’ll work exactly the same way.”
Different experiments might require different types of processing, Tejaswi says. For example, some experimental systems require acid-washed glassware, while other glassware must never be exposed to any kind of detergent. For some special applications, the glassware must be baked at high temperatures for over eight hours before it can be used. In all cases, the cleaning protocols are carefully followed.
“The team is very motivated because every speck, every dot matters,” Tejaswi says. The washers check for quality at every step of the process, including one more time while they’re carting the freshly cleaned materials to the hallway shelves. “Before they shelve it, they’ll still catch one more impurity out of 100 pieces of glassware,” she says. “That’s how accurate they are.”
“The faculty really value these services, and some of them have been at other institutions where the services are not even remotely as good,” says Brooke Bevis, the Glasswashing Core manager. “They already understand how important glasswashing is, and they’re really good at teaching and demonstrating to their lab members that you need to treat people who are providing a service to you with respect.”
Tejaswi came to Whitehead Institute last year. As the team’s supervisor, she focuses on servicing machinery, taking care of the chemical room, ordering materials, and coordinating with the researchers. She also fills in as a glasswasher when needed. “I have a good team,” she says. “They know what they have to do, and they’re teaching me.”
The lab floors of the building all have a glasswashing room at the north end of the floor, meaning that Tejaswi often has to run between floors to coordinate her team’s efforts. “Jumping up and down between the different floors is fun,” she says. “It keeps things active.”
The quality of the community at Whitehead Institute has stood out to Tejaswi since she arrived. “Everybody is so nice and friendly here,” she says. “I haven’t seen such a friendly place that caters to employees as humans so much before. It takes so much of the pressure off. Then it’s just your own work ethic and priorities that you have to worry about, and you reach your goals with much more ease and happiness than if you are getting a lot of pressure from others.”
Bevis says that while dishwashing machines aid the team’s efficiency, many things have to be processed by hand. “This is one of those things that can’t really be automated or sped up,” she says. “These guys really, really take pride in the work that they’re doing, with a lot of attention to detail.”
“It’s a little territorial at times—in a good way,” Tejaswi says. “It’s like, ‘This is mine, I have to do it this way.’ They take ownership of their roles. Then they double-check each other.”
Outside of work, Bevis spends time with her kids and enjoys reading and going to movies. Tejaswi is part of a classical Indian vocal group, teaches singing lessons, and volunteers at Hindu temples in the Boston area. About once a month, the glasswashing team gets brunch together.
Eric Spooner
Proteomics Core Facility
 |
| Proteomics Core Facility Director Eric Spooner Credit: Conor Gearin/Whitehead Institute |
Eric Spooner knows his way around a protein. “I started in this field before proteomics became a word,” he says. With over 20 years of experience with analyzing proteins, most of that as facility manager of the Proteomics Core Facility at Whitehead Institute, Spooner aids researchers looking to measure protein levels in cells, figure out how a complex fits together, or evaluate how different proteins interact with each other and their surroundings.
Though there are tools for looking at RNA transcripts to measure gene expression, Spooner says it’s often important to go all the way to protein levels to know what’s really going on in the cell. “The quantities don’t always match up between the levels of RNA in the cell and the proteins in the cell,” he says. “There’s protein turnover that occurs. Some proteins are long-lived, some are short-lived.”
What’s more, many drugs affect their targets by changing a protein’s shape. “If you can add a drug that latches onto a protein and changes its 3D structure to where it can’t do its job efficiently anymore, that would never show up in the RNA transcripts,” he says.
For measuring relative levels of a protein in a treated group of cells compared to a control group, Spooner uses techniques such as SILAC or iTRAQ that involve labelling amino acids or peptides with isotopes of carbon or nitrogen that have an extra neutron (13C and 15N). A high-resolution mass spectrometer lets him measure the relative difference between the treated and untreated cells.
The Proteomics Core’s set of mass spectrometry tools let researchers tease out questions about what happens to proteins after the RNA transcript is translated into a sequence of peptides. Many proteins undergo post-translational processing in which peptides are added or taken away. Spooner can also use a cross-linking analysis to figure out how the different subunits of a protein complex fit together. “It goes hand-in-hand with X-ray diffraction analysis for protein structure,” he says.
Spooner appreciates the collegial atmosphere at Whitehead Institute. “I like that I have the independence to kind of run my own show here,” he says. “I’m trusted to make good decisions and keep the core running. There’s no rigorous protocol that they have to follow to come and talk to me. That’s just how I like to run things.”
Many of the administrative hurdles faced by larger institutions don’t exist here, he says. “It’s a lot easier here, because we all know each other,” he says. “At a larger place, you’re just another name on a list.”
Spooner is a music fan and a sustaining member of WERS, the Emerson College independent radio station that he usually tunes into in the lab. Lately, he and his wife has gone to shows by blues and rock musicians including Joe Bonamasa and Dweezil Zappa, Broadway star Barbra Streisand, and the jazz vocal group Manhattan Transfer. He recently moved from Somerville to Dorchester, and has been exploring the restaurant scene in his new neighborhood.
Nicki Watson and Wendy Salmon
W. M. Keck Microscopy Facility
 |
| Lab Manager and Electron Microscopy Specialist Nicki Watson (left) and Light Microscopy Specialist Wendy Salmon (right) Credit: Conor Gearin/Whitehead Institute |
Light Microscopy Specialist Wendy Salmon sometimes finds herself looking into microscopy images like shapes in the clouds. “I’ll say, ‘Look, there’s a snowman!’” she says. The habit comes a deep familiarity with the odd shapes found in microscopy, she says.
Lab Manager and Electron Microscopy Specialist Nicki Watson explains that these imaginative descriptions help communicate with researchers while exploring their images in the W. M. Keck Microscopy Facility. “If you looking through the oculars of the microscope, and you’re trying to describe what you’re seeing to someone who’s not looking, you don’t know if you’re looking at the same thing,” Watson says. “You get creative in trying to describe what you’re seeing.”
Since it takes a long time to learn electron microscopy techniques, Watson is able to save researchers time. “If they only need 3 or 4 pictures for one paper, it’s not worth their six months to train to get competent enough to produce publication-quality work,” she says. However, if someone’s paper or thesis focuses on electron microscopy, she helps train them on the equipment.
Similarly, Salmon tries to get researchers as independent as they can be if their light microscopy protocols are relatively straightforward. “It also makes them much more adept at navigating the results of their experiments,” Salmon says. “When they understand the instrumentation better, they can interpret what they’re seeing and make decisions on their own.”
Watson recalls a particularly satisfying project in which she achieved something she did not think was possible. A researcher was looking for a tiny developing kidney in an embryonic zebrafish, but was unsure what it would look like — making it difficult to locate. Doubtful at first, Watson searched through section after section. “I kept at it, looking and looking, and finally saw something,” she says. “Not surprisingly, it looks like a kidney, with glomeruli and tubules.”
Sometimes, microscopy can reveal surprises. Salmon once assisted researchers trying to make time-lapse images of mammary organoids to see if the organoid’s structure would change over time. When the team played back the overnight time-lapse, they saw something unexpected — cells were roaming around within the larger structure, and the video clearly captured their journeys.
Microscopy brings a unique perspective to research projects, Salmon says. “Almost everything else is a bulk measurement of some sort,” she says. “With microscopy you know where something is happening. You get spatial context.”
“That’s true right down to the ultrastructural level on the electron microscope, too,” Watson agrees. “In electron microscopy, you can look at how these perturbations are affecting the ultrastructure of the cell, at a high-resolution level.”
Maya Mitalipova
Human Stem Cell Facility
 |
| Director of the Human Stem Cell Facility, Maya Mitalipova Credit: Conor Gearin/Whitehead Institute |
When Whitehead Institute researchers take on a stem cell experiment, they are able to collaborate with one of the first scientists to work in the field. Maya Mitalipova, director of the Human Stem Cell Facility, isolated some of the first human embryonic stem cell lines confirmed by the National Institutes of Health in 2001. With 18 years of experience with human embryonic stem cells (HSCs) and having isolated 19 HSC lines at Whitehead Institute, Mitalipova says that her knowledge lets her troubleshoot nearly any hurdle that researchers encounter.
“I think that can only be done by people that have been there since the beginning of the field,” she says. “That’s what makes my position unique.”
Mitalipova grew up in Kazakhstan and completed her undergraduate and PhD studies in Russia, carrying out pathbreaking stem cell research with mice. Following postdoctoral work with Neal First at the University of Wisconsin-Madison, she moved to Athens, Georgia, to isolate some of the first human stem cells for BresaGen, Inc., with the goal of studying and treating Parkinson’s disease. But while working for the company, Mitalipova missed the ability to ask big research questions. She began interviewing for academic positions. She chose to be a facility director at Whitehead Institute rather than a faculty position elsewhere because of the chance to collaborate with the researchers here, she says.
“I can’t imagine my life without Whitehead,” Mitalipova says. “I’d never regret that I didn’t take some other position elsewhere, because of how much I’ve learned here. It doesn’t matter what title you have. What is more important for me is the science that I can do, the place I work, and the people around me.”
In her role as facility director, Mitalipova consults with researchers before experiments begin to establish the strategy. If a postdoctoral researcher’s study will focus on stem cell biology, the researcher joins her team to train extensively before they begin experiments of their own. Mitalipova enjoys learning from new team members about research techniques she hasn’t experienced yet. “I am as excited as 14 years ago,” she says. “I’m still learning.”
Improvements in induced pluripotent stem cell (iPSC) methods allow scientists to take cells from a patient with a disease, transform the cells back into an undifferentiated state, and then grow live tissue to study in the lab. Human embryonic stem cells (HSCs) let Mitalipova figure out how to guide the iPSCs to differentiate into the cell type of interest — for example, into neurons to study neurodegeneration. Once she and her collaborators decide on the differentiation protocol, they apply it to patient iPSCs to study the disease phenotype.
Over the years, Mitalipova has continued isolating new HSC lines for use in research, as well as developing hundreds of iPSC lines. “Our human ES cell lines have been highly desirable to the research community around the world, which makes me feel pretty good,” she says. “The best-known labs around the world working with HSCs and iPSCs have our lines.”
Stem cells are very picky about their environment, Mitalipova says. Experiments that differentiate stem cells into mature tissue run relatively long — often six months or more. The unique challenges of stem cell biology lead Mitalipova to make herself available throughout the week. “If researchers have trouble, they have my cell number and can ask me questions,” she says. They’ll ask if I’ll be in the lab, and I am in the lab 7 days a week — because the cell cultures don’t have weekends or holidays.”
Mitalipova’s weekend companion in the lab for many years was her mother. Maya’s passion for researching neurodegenerative diseases became even stronger when her mother was diagnosed with Parkinson’s and Maya began taking care of her.
“Because I had a Parkinson’s patient in my hand, and I was her only caregiver, I understood that disease from the patient perspective as well as from the researcher perspective,” she says. “When you lose the closest person in your life to this disease, you’re very dedicated.” Her mother passed away 18 months ago.
“I realized that it was a gift that I was able to take care of my mom and still keep my job,” Maya says. Her mother knew everything about her daughter’s work in the lab. “Just spending time with my mom, who I miss so much already, was really important to me,” she says. “The best times would be when I would take her to the beach, and she would be so happy to watch the sea. I miss that.”
In her free time, Mitalipova has become an advocate for the human rights of Uyghurs, the ethnic group from Central Asia to which she belongs. “With their families in danger, they have no way to defend themselves. As an American, I can raise my voice to help,” she says.
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


