Elegant switch controls translation in transition from egg to embryo
The transition from an egg to a developing embryo is one of life’s most remarkable transformations. Whitehead Institute researchers have deciphered how one aspect—control of the all-important translation of messenger RNAs (mRNAs) into proteins—switches as the egg becomes an embryo. That shift is controlled by a beautiful mechanism, which is triggered at a precise moment in development and automatically shuts itself off after a narrow window of 20 to 90 minutes.
CAMBRIDGE, Mass. – The transition from an egg to a developing embryo is one of life’s most remarkable transformations. Yet little is known about it. Now Whitehead Institute researchers have deciphered how one aspect—control of the all-important translation of messenger RNAs (mRNAs) into proteins—switches as the egg becomes an embryo. That shift is controlled by a beautiful mechanism, which is triggered at a precise moment in development and automatically shuts itself off after a narrow window of 20 to 90 minutes.
As an egg develops, it stockpiles mRNAs from the mother because it will not have time to create new mRNAs during the rapid development of a very early embryo. When fertilized the egg becomes an embryo, the stashed maternal mRNAs are pressed into service for a brief window before the embryo starts transcribing its own mRNAs. This change occurs very early; in humans, only two to four cell divisions occur before this transition is executed. Whitehead Member Terry Orr-Weaver studies the control of translation of maternal mRNAs in the model organism Drosophila, or the fruit fly, because its developmental strategy offers experimental advantages.
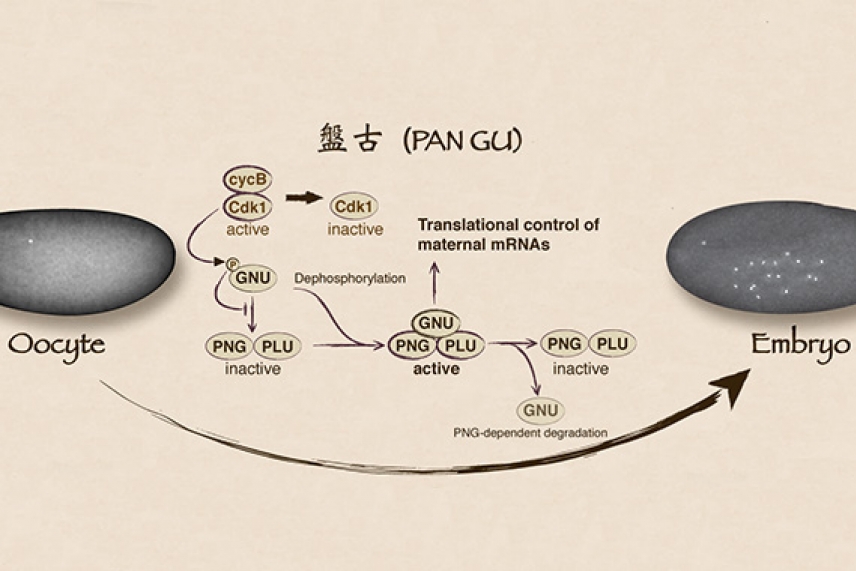
In the current research, Orr-Weaver and her lab determined that key to the transition are the three molecules that form the enzyme PNG kinase: PNG, PLU, and GNU. Orr-Weaver describes PNG and PLU as “tight buddies” that are always locked together, including in the mature egg. At that point in development, GNU has phosphate molecules tacked to it, which impede its binding to PNG-PLU.
When an egg is activated, levels of another enzyme that adds phosphates to GNU in the egg precipitously drop, allowing GNU to lose its phosphates and bind to PNG-PLU. Once together, the trio comprises the PNG kinase that triggers the translational control of the maternal mRNAs. Because PNG kinase also triggers the break down of GNU, the kinase self-destructs, which quickly and irreversibly squelches the translation of maternal mRNAs. This elegant feedback loop and the switch it controls are described in an article in eLife.
The design of this transition could tell scientists more about how human cells work and embryos develop. For example, the switch could be a model for how cells massively and globally change mRNA translation. Also, similar kinase activity during early development has been noted in worms, which may mean that a comparable approach is used in other organisms, including humans.
Although she now has as greater understanding of how translation is turned on and off during very early embryonic development, Orr-Weaver is intrigued by how the switch is linked to the translation machinery.
“PNG is a kinase, which means it controls things by what it phosphorylates,” says Orr-Weaver, who is also an American Cancer Society Research Professor of biology at MIT. “So the big question is what proteins does it phosphorylate? Finding that out is the next big phase of this project.”
This work was supported by the Japan Society for the Promotion of Science, the Uehara Memorial Foundation, and the National Institutes of Health (NIH grants GM39341 and GM118098).
* * *
Terry Orr-Weaver’s primary affiliation is with Whitehead Institute for Biomedical Research, where her laboratory is located and all her research is conducted. She is also an American Cancer Society Research Professor of biology at the Massachusetts Institute of Technology.
* * *
Citation
Hara, Masatoshi, Boryana Petrova, and Terry L. Orr-Weaver. Control of PNG kinase, a key regulator of mRNA translation, is coupled to meiosis completion at egg activation. eLife 6 (2017): e22219.
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


