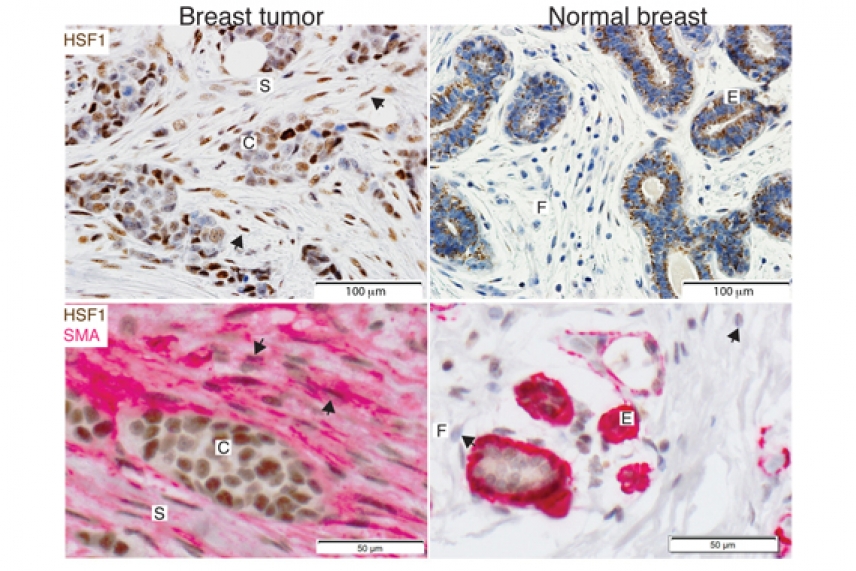
HSF1 is activated in cancer associated fibroblasts (CAFs) within the tumor microenvironment of breast cancer patients. Tissue sections from breast cancer patient biopsies encompassing both invasive carcinoma (Left panels) and normal breast lobules (Right panels) were immunostained to detect HSF1 (brown, upper panels). Costaining with a marker for CAFs (Pink, lower panels) confirmed the activation of HSF1 in these cells. Arrows indicate HSF1-positive CAFs in the left panels and normal, HSF1-negetive fibroblasts in the lower right panel.
Courtesy of Cell
Master heat-shock factor supports reprogramming of normal cells to enable tumor growth and metastasis
CAMBRIDGE, Mass. – Long associated with enabling the proliferation of cancer cells, the ancient cellular survival response regulated by Heat-Shock Factor 1 (HSF1) can also turn neighboring cells in their environment into co-conspirators that support malignant progression and metastasis.
The finding, reported by Whitehead Institute scientists this week in the journal Cell, lends new insights into tumor biology with significant implications for the diagnosis, prognosis, and management of cancer patients.
Over the past several years, researchers in the lab of Whitehead Member Susan Lindquist have been investigating the role the transcription factor HSF1 plays in supporting malignancy. In normal cells, stressful conditions, including those caused by heat, hypoxia, and toxins activate HSF1, which serves to maintain protein homeostasis and helps the cells endure tough times. Cancer cells, however, are capable of hijacking this heat-shock response to their own benefit. Two years ago, Lindquist’s lab implicated HSF1 in this corruption, showing that it activates a set of genes in cancer cells quite distinct from those up-regulated in normal cells during heat-shock.
Building upon that research, the lab has now discovered that HSF1 operates not only on the cancer cells in a tumor, but also on the cells of the tumor microenvironment, or stroma. Here HSF1 drives a transcriptional program distinct from that operating in adjacent cancer cells. HSF1 activation in both cancer cells and stromal cells is a powerful, complementary combination that fuels malignant processes.
“This is actually a beautiful example of evolution,” says Ruth Scherz-Shouval, a postdoctoral scientist in the Lindquist lab and first author of the Cell paper. “It’s recognizing that the tumor is like an organism that adheres to evolutionary principles. HSF1 has been highly conserved over time, supporting the survival of organisms ranging from yeast to human, so it makes sense that it is co-opted here. Both cancer cells and the microenvironment are sensing changes in the tumor and responding, signaling to one another to help the “organism”, albeit to the detriment of the host. These are different programs, but they’re both controlled by HSF1 and serve the same purpose.”
In a series of experiments, Scherz-Shouval and colleagues found clear evidence of HSF1 activation in stromal cells known as cancer-associated fibroblasts, or CAFs, in a variety of human tumors, including breast, lung, skin, esophageal, colon, and prostate cancers. Moreover, they discovered that not only does HSF1 activation in CAFs up-regulate genes supporting malignancy, it also suppresses genes that would ordinarily trigger a protective, anti-cancer immune response in surrounding tissue. Although such a synergistic dynamic may seem daunting to overcome, it may in fact actually present a real opportunity for therapeutic intervention.
“It’s important to find HSF1 operating this way in the stroma,” notes Scherz-Shouval. “The tumor microenviroment tends to be more genetically stable and less prone to mutation, suggesting that even if cancer cells could mutate to evade therapeutic disruption of HSF1, supportive cells in the stroma could still be susceptible.”
“Although it’s thought to be quite difficult to drug a transcription factor like HSF1 directly, the role of HSF1 suggests that we might be able to treat cancer more effectively by modifying the underlying tumor biology,” says Luke Whitesell, an oncologist, Lindquist lab senior scientist, and a corresponding author on the latest Cell paper. “Targeting the dual role of HSF1 has the potential to change how a cancer responds to therapeutic interventions, perhaps making it less able to cope with other therapies.”
Another significant finding from the research is the potential to use stromal HSF1 activation as a diagnostic and prognostic biomarker. In analysis of tumor samples from breast cancer patients, the scientists found that HSF1 activation in the stroma was associated with poor patient outcomes, including reduced disease-free survival and overall survival. Further, the researchers also found that stromal HSF1 activation in samples from patients with early-stage non-small cell lung cancer was also associated with poor outcomes.
Although the scientists emphasize that the numbers of breast and lung cancer samples studied were small, the correlation between stromal HSF1 activation and poor patient outcomes was strong enough in each case to warrant further clinical investigation. They add that an HSF1-based biomarker could help predict which patient tumors, early-stage lung in particular, are most likely to progress and might benefit from more aggressive therapy. Conversely, such information could prevent patients with less aggressive cancers from suffering the ill effects of “over-treatment” with highly toxic therapies.
This work is supported by the National Institutes of Health and the National Cancer Institute (grant K08 NS064168 and K99CA175293), the Howard Hughes Medical Institute, the V Foundation, the Komen Foundation, the Human Frontiers Science Program, the Fulbright Program, the Jared Branfman Sunflowers for Life Fund, the Israel National Postdoctoral Award Program for Women in Science, and the J & J COSAT focused funding program.
***
Susan Lindquist’s primary affiliation is with Whitehead Institute for Biomedical Research, where her laboratory is located and all her research is conducted. She is also a Howard Hughes Medical Institute investigator and a professor of biology at Massachusetts Institute of Technology.
***
Citation:
Scherz-Shouval, R., Santagata, S., Mendillo, M., Sholl, L., Ben-Aharon, I., Beck, A., . . . Lindquist, S. (2014). The Reprogramming of Tumor Stroma by HSF1 Is a Potent Enabler of Malignancy. Cell, 158(3), 564-578. doi:10.1016/j.cell.2014.05.045
Topics
Contact
Communications and Public Affairs
Phone: 617-452-4630
Email: newsroom@wi.mit.edu


